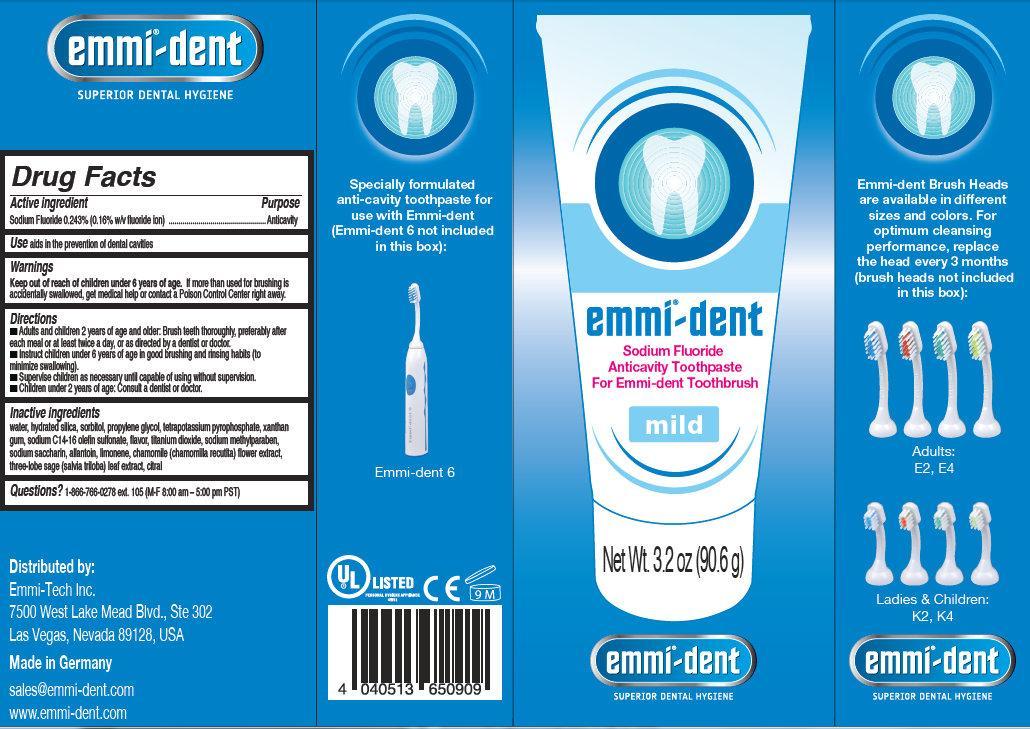
Directions
- Adults and children 2 years and older: Brush teeth thoroughly, preferably after each meal or at least twice a day, or as directed by a dentist or doctor.
- Instruct children under 6 years of age in good brushing and rinsing habits (to minimize swallowing).
- Supervise children as necessary until capable of using without supervision.
- Children under 2 years: Consult a dentist or doctor.
Inactive Ingredients
water, hydrated silica, sorbitol, propylene glycol, tetrapotassium pyrophosphate, xanthan, gum, sodium C14-16 olefin sulfonate, flavor, titanium dioxide, sodium methylparaben, sodium saccharin, allantoin, limonene, chamomile (chamomilla recutita) flower extract, three-lobe sage (salvia triloba) leaf extract, citral