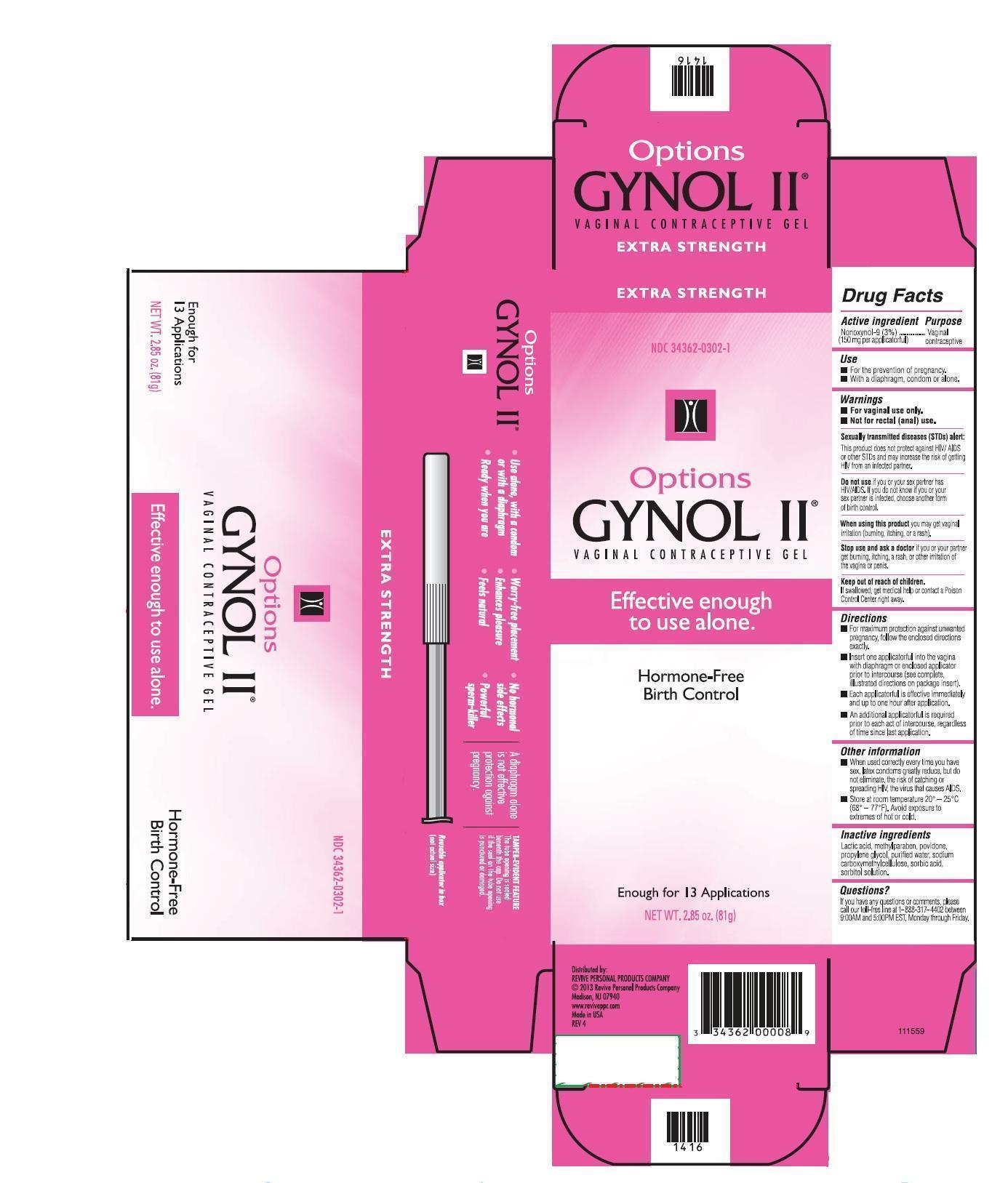
Active ingredient
Nonoxynol-9 (3%) (100 mg per applicatorful)............................................Vaginal Contraceptive
For vaginal use only.
Not for rectal (anal) use.
Sexually transmitted diseases (STDs) alert:
This product does not protect against HIV/AIDS or other STDs and may increase the risk of getting HIV from an infected partner.
Do not use if you or your sex partner has HIV/AIDS. If you do not know if your sex partner is infected, choose another form of birth control.
Stop use and ask a doctor if you or your partner gets burning, itching, a rash, or other irritation or the vagina or penis.
Directions
For maximum protection against unwanted pregnancy, follow the enclosed directions exactly.
Each applicatorful is effective immediately and up to one hour after application.
Insert one applicatorful into the vagina prior to intercourse (see complete, illustrated directions on the package insert).
An additional applicatorful is required prior to each act of intercourse, regardless of time since last application.
Other Information
When used correctly every time you have sex, latex condoms greatly reduce, but do not eliminate, the risk of catching or spreading HIV, the virus that causes AIDS.
Store at room temperature, 20° - 25° C (68° -77° F). Avoid exposure to extremes of hot or cold.