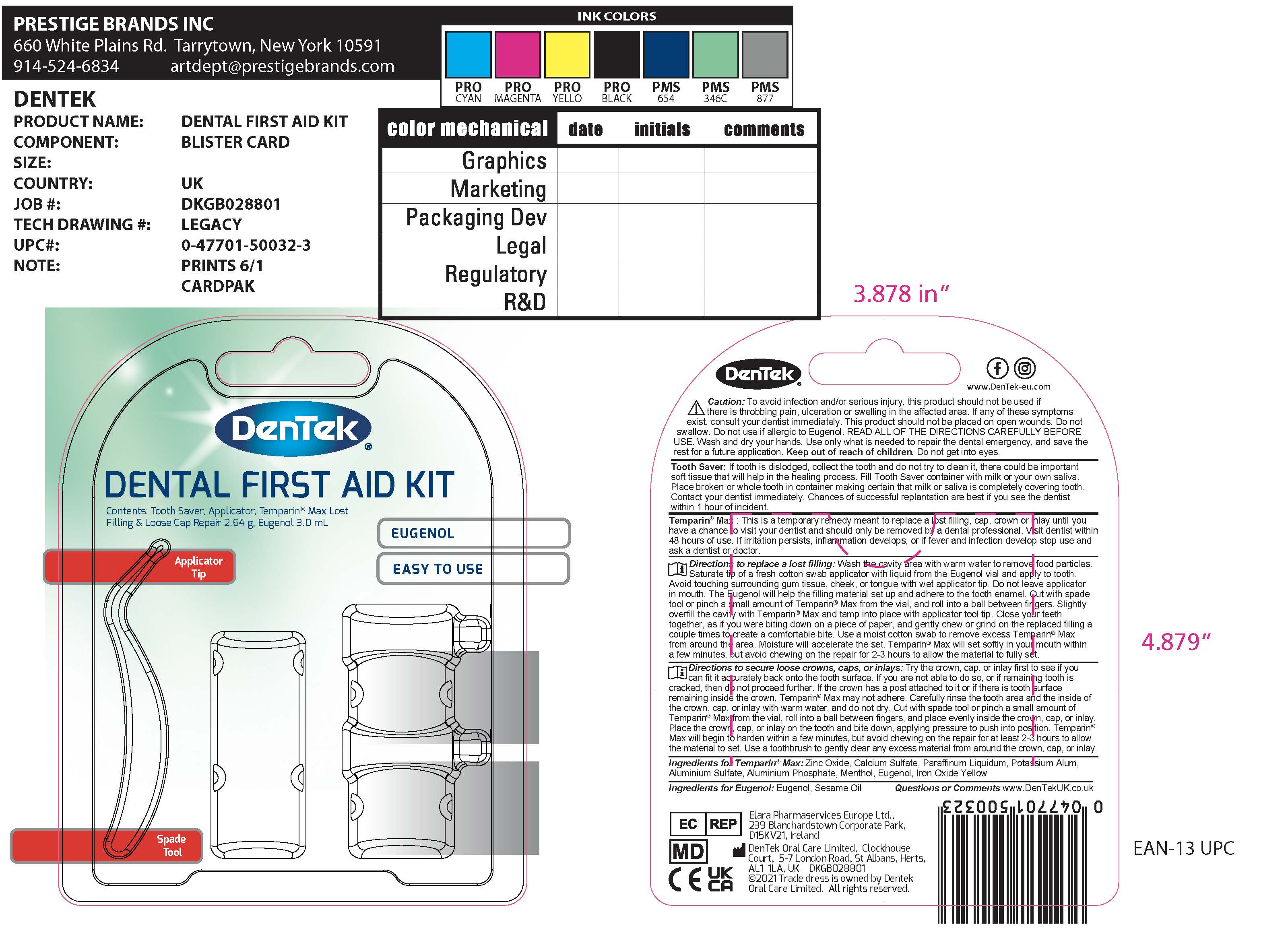
Uses
For the temporary relief of throbbing, persisitent toothache due to a cavity. Visit Dentist within 48 hours of use.
Warnings
Allergy alert: do not use if you are allergic to eugenol (clove oil).
When using this product
- use only in teeth with persistent throbbing pain
- avoid touching tissuers other than tooth cavity
- DO NOT SWALLOW to avoid irritation
- avoid contact with eyes.
Directions
Directions Adults and children 12 year of age and older: Rinse the tooth with water to remove any food particles from the cavity. Moisten a cotton swab with Eugenol and place in the cavity for approximately 1 minute. Avoid touching tissue other than the tooth cavity. Apply the dose not more than four times daily or as directed by dentist or physician.
Adults and children 12 years and older use up to 4 times daily or as directed by a dentist or doctor.
Children under 12 years ask a dentist or doctor.