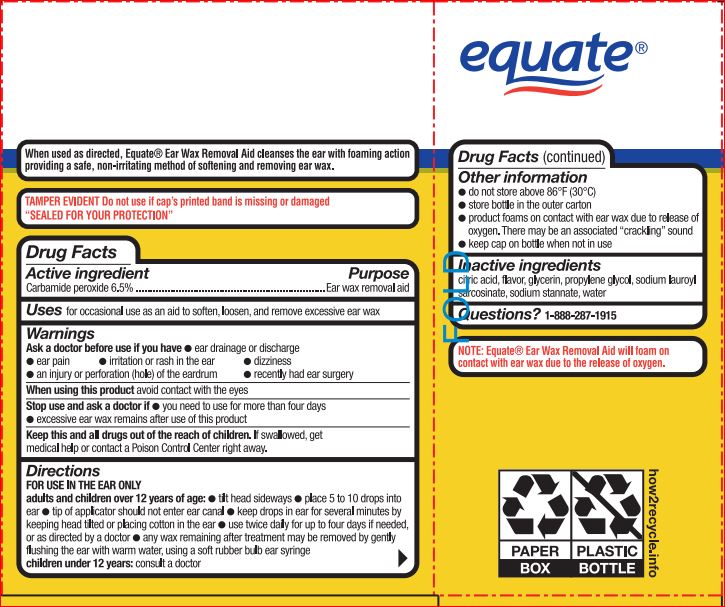
Warnings
For external use only
Keep out of reach of the children
If product is swallowed, get medical help or contact a Poison Control Center right away
Directions For use in the ear only.
Adults and children over 12 years of age:
- tilt head sideways and place 5 to 10 drops into ear
- tip of applicator should not enter ear canal
- keep drops in ear for several minutes by keeping head tilted or placing cotton in the ear
- use twice daily for up to 4 days if needed, or as directed by a doctor
- any wax remaining after treatment may be removed by gently flushing the ear with warm water, using a soft rubber bulb ear syringe
Children under 12 years of age: consult a doctor.