FULL PRESCRIBING INFORMATION
WARNING: THROMBOSIS
- •
- Serious arterial and venous thrombotic events following administration of NOVOSEVEN RT have been reported. [See Warnings and Precautions (5.1)]
- •
- Discuss the risks and explain the signs and symptoms of thrombotic and thromboembolic events to patients who will receive NOVOSEVEN RT. [See Warnings and Precautions (5.1)]
- •
- Monitor patients for signs or symptoms of activation of the coagulation system and for thrombosis. [See Warnings and Precautions (5.1)]
1 INDICATIONS AND USAGE
NOVOSEVEN RT, Coagulation Factor VIIa (Recombinant), is indicated for:
- •
- Treatment of bleeding episodes and peri-operative management in adults and children with hemophilia A or B with inhibitors, congenital Factor VII (FVII) deficiency, and Glanzmann’s thrombasthenia with refractoriness to platelet transfusions, with or without antibodies to platelets.
- •
- Treatment of bleeding episodes and peri-operative management in adults with acquired hemophilia.
2 DOSAGE AND ADMINISTRATION
For intravenous administration only
2.1 Dose
- •
- Use hemostasis evaluation to determine the effectiveness of NOVOSEVEN RT and to provide a basis for modification of the NOVOSEVEN RT treatment schedule.
- •
- Coagulation parameters do not necessarily correlate with or predict the effectiveness of NOVOSEVEN RT.
Treatment of Acute Bleeding Episodes
NOVOSEVEN RT dosing for the treatment of acute bleeding episodes is provided in Table 1.
Table 1: Dosing for Treatment of Acute Bleeding Episodes
| Dose* and
Frequency | Duration of Therapy | Additional Information |
|---|---|---|
|
||
|
Congenital Hemophilia A or B with Inhibitors |
||
|
Hemostatic 90 mcg/kg every two hours, adjustable based on severity of bleeding Post-Hemostatic 90 mcg/kg every 3-6 hours for severe bleeds |
Until hemostasis is achieved, or until the treatment has been judged to be inadequate After hemostasis is achieved to maintain the hemostatic plug |
The appropriate duration of post-hemostatic dosing has not been studied |
|
Acquired Hemophilia |
||
|
70-90 mcg/kg every 2-3 hours |
Until hemostasis is achieved | |
|
Congenital Factor VII Deficiency |
||
|
15-30 mcg/kg every 4-6 hours |
Until hemostasis is achieved |
Effective treatment has been achieved with doses as low as 10 micrograms per kg body weight. Adjust dose and frequency of injections to each individual patient |
|
Glanzmann’s Thrombasthenia |
||
|
90 mcg/kg every 2-6 hours |
In severe bleeding episodes requiring systemic hemostatic therapy until hemostasis is achieved |
Platelet transfusions are the primary treatment in patients with Glanzmann’s Thrombasthenia without refractoriness to platelets or in patients without platelet-specific antibodies |
Congenital Hemophilia A or B with inhibitors
- •
- Dose and administration interval may be adjusted to the individual patient based on the severity of the bleeding.1
- •
- For patients treated for joint or muscle bleeds, a decision on outcome was reached for a majority of patients within eight doses although more doses were required for severe bleeds. A majority of patients who reported adverse experiences received more than twelve doses. Monitor and minimize the duration of any post-hemostatic dosing.
Perioperative Management
NOVOSEVEN RT dosing for prevention of bleeding in surgical interventions or invasive procedures (perioperative management) is provided in Table 2.
Table 2: Dosing for Perioperative Management
| Type of Surgery | Dose and Frequency | Additional Information |
|---|---|---|
|
||
|
Congenital Hemophilia A or B with Inhibitors |
||
|
Minor |
Initial:
Post surgical: 90 mcg/kg every 2 hours for 48 hours then every 2-6 hours until healing occurs | |
|
Major |
Initial: 90 mcg/kg immediately before surgery and repeat every 2 hours for the duration of the surgery Post surgical: 90 mcg/kg every 2 hours for 5 days then every 4 hours or by continuous infusion at 50 mcg/kg/hr until healing occurs |
Additional bolus doses can be given |
|
Acquired Hemophilia |
||
|
Minor or Major |
70-90 mcg/kg immediately before surgery and repeat every 2-3 hours for the duration of the surgery and until hemostasis is achieved* | |
|
Congenital Factor VII Deficiency |
||
|
Minor or Major |
15-30 mcg/kg immediately before surgery and repeat every 4-6 hours for the duration of the surgery and until hemostasis is achieved* Adjust dose and frequency of injections to each individual patient |
Doses as low as 10 micrograms per kg body weight can be effective |
|
Glanzmann’s Thrombasthenia |
||
|
Minor or Major |
Initial: 90 mcg/kg immediately before surgery and repeat every 2 hours for the duration of the procedure* Post surgical: 90 mcg/kg every 2-6 hours to prevent post-operative bleeding* |
Higher doses of 100-140 micrograms per kg can be used for surgical patients who have clinical refractoriness with or without platelet-specific antibodies |
2.2 Reconstitution
- •
- Follow the procedures below for the preparation and reconstitution of NOVOSEVEN RT. For questions regarding reconstitution, please contact Novo Nordisk at 1-877-NOVO-777.
- •
- Calculate the NOVOSEVEN RT dosage and select the appropriate NOVOSEVEN RT package provided with either 1 histidine diluent vial or 1 pre-filled histidine diluent syringe.
- •
- Reconstitute only with the histidine diluent provided with NOVOSEVEN RT.
NOVOSEVEN RT package containing 1 vial of NOVOSEVEN RT powder and 1 vial of histidine diluent:
- 1.
- Always use aseptic technique.
- 2.
- Bring NOVOSEVEN RT (white, lyophilized powder) and the specified volume of histidine (diluent) to room temperature, but not above 37°C (98.6° F). The specified volume of diluent corresponding to the amount of NOVOSEVEN RT is as follows:
1 mg (1000 micrograms) vial + 1.1 mL Histidine diluent
2 mg (2000 micrograms) vial + 2.1 mL Histidine diluent
5 mg (5000 micrograms) vial + 5.2 mL Histidine diluent
8 mg (8000 micrograms) vial + 8.1 mL Histidine diluent - 3.
- Remove caps from the NOVOSEVEN RT vials to expose the central portion of the rubber stopper. Cleanse the rubber stoppers with an alcohol swab and allow to dry prior to use.
- 4.
- Draw back the plunger of a sterile syringe (attached to sterile needle) and admit air into the syringe. It is recommended to use syringe needles of gauge size 20-26.
- 5.
- Insert the needle of the syringe into the Histidine diluent vial. Inject air into the vial and withdraw the quantity required for reconstitution.
- 6.
- Insert the syringe needle containing the diluent into the NOVOSEVEN RT vial through the center of the rubber stopper, aiming the needle against the side so that the stream of liquid runs down the vial wall (the NOVOSEVEN RT vial does not contain a vacuum). Do not inject the diluent directly on the NOVOSEVEN RT powder.
- 7.
- Gently swirl the vial until all the material is dissolved. The reconstituted solution is a clear, colorless solution which may be stored either at room temperature or refrigerated for up to 3 hours after reconstitution. After reconstitution with the specified volume of diluent, each vial contains approximately 1 mg per mL NOVOSEVEN RT (1000 micrograms per mL).
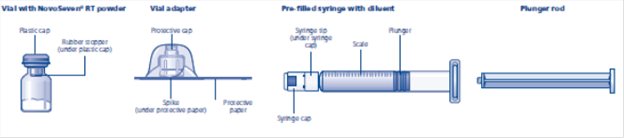
NOVOSEVEN RT package containing 1 vial of NOVOSEVEN RT powder and 1 pre-filled histidine diluent syringe with vial adapter for needleless reconstitution:
- 1.
- Always use aseptic technique.
- 2.
- Bring NOVOSEVEN RT (white, lyophilized powder) and the specified volume of histidine (diluent) to room temperature, but not above 37°C (98.6° F). The specified volume of diluent corresponding to the amount of NOVOSEVEN RT is as follows:
1 mg (1000 micrograms) vial + 1 mL Histidine diluent in pre-filled syringe
2 mg (2000 micrograms) vial + 2 mL Histidine diluent in pre-filled syringe
5 mg (5000 micrograms) vial + 5 mL Histidine diluent in pre-filled syringe
8 mg (8000 micrograms) vial + 8 mL Histidine diluent in pre-filled syringe - 3.
- Remove cap from the NOVOSEVEN RT vial. Cleanse the rubber stopper with an alcohol swab and allow to dry prior to use.
- 4.
- Peel back the protective paper from the vial adapter. Do not remove the vial adapter from the package.
- 5.
- Place the NOVOSEVEN RT vial on a flat surface. While holding the vial adapter package, place the vial adapter over the NOVOSEVEN RT vial and press down firmly on the package until the vial adapter spike penetrates the rubber stopper.
- 6.
- Attach the plunger rod to the syringe. Turn the plunger rod clockwise into the plunger inside the pre-filled diluent syringe until resistance is felt. Remove the syringe cap from the pre-filled diluent syringe and screw onto the vial adapter.
- 7.
- Push the plunger rod to slowly inject all the diluent into the vial. Keep the plunger rod pressed down and swirl the vial gently until the powder is dissolved. The reconstituted solution is a clear, colorless solution which may be stored fully assembled either at room temperature or refrigerated for up to 3 hours after reconstitution. After reconstitution with the specified volume of diluent, each vial contains approximately 1 mg per mL NOVOSEVEN RT (1000 micrograms per mL).
2.3 Administration
For intravenous injection only
- •
- Inspect the reconstituted NOVOSEVEN RT visually for particulate matter and discoloration prior to administration, whenever solution and container permit. Do not use if particulate matter or discoloration is observed.
- •
- Do not freeze reconstituted NOVOSEVEN RT or store it in syringes.
- •
- Administer within 3 hours after reconstitution.
- •
- Do not mix with other infusion solutions.
- •
- Discard any unused solution.
Perform the following procedures immediately prior to administration:
NOVOSEVEN RT package containing 1 vial of NOVOSEVEN RT powder and 1 vial of histidine diluent:
- 1. Always use aseptic technique.
- 2. Draw back the plunger of a sterile syringe (attached to sterile needle) and admit air into the syringe.
- 3. Insert needle into the vial of reconstituted NOVOSEVEN RT. Inject air into the vial and then withdraw
- the appropriate amount of reconstituted NOVOSEVEN RT into the syringe.
- 4. Remove and discard the needle from the syringe.
NOVOSEVEN RT package containing 1 vial of NOVOSEVEN RT powder and 1 pre-filled histidine diluent syringe with vial adapter for needleless reconstitution:
- 1. Always use aseptic technique.
- 2. Invert the NOVOSEVEN RT vial. Stop pushing the plunger rod and let it move back on its own while
- the mixed solution fills the syringe. Pull the plunger rod slightly downwards to draw the mixed solution into the syringe. Tap the syringe to remove air bubbles and withdraw the required dose amount of reconstituted NOVOSEVEN RT into the syringe.
- 3. Unscrew the vial adapter with the vial. Discard the empty NOVOSEVEN RT vial with the vial adapter
- attached.
Caution:
- •
- The pre-filled diluent syringe is made of glass with an internal tip diameter of 0.037 inches, and is compatible with a standard Luer-lock connector.
- •
- Some needleless connectors for intravenous catheters are incompatible with the glass diluent syringes (for example, certain connectors with an internal spike, such as Clave® /MicroClave®, InVision-Plus®, InVision-Plus CS®, InVision-Plus® Junior®, Bionector®), and their use can damage the connector and affect administration. To administer product through incompatible needleless connectors, withdraw reconstituted product into a standard 10 mL sterile Luer-lock plastic syringe.
- •
- If you have encountered any problems with attaching the pre-filled histidine diluent syringe to any Luer lock compatible device, please contact Novo Nordisk at (877) 668-6777.
Administer NOVOSEVEN RT bolus infusion using the following procedures:
- 1. Administer as a slow bolus injection over 2 to 5 minutes, depending on the dose administered.
- 2. If line needs to be flushed before or after NOVOSEVEN RT administration, use 0.9% Sodium Chloride
- Injection, USP.
- 3. Discard any unused reconstituted NOVOSEVEN RT after 3 hours.
Administer NOVOSEVEN RT continuous infusion for perioperative management using the following procedures:
- 1.
- Administer as a continuous infusion at 50 mcg/kg/hr using an infusion pump.
- 2.
- If line needs to be flushed before or after NOVOSEVEN RT administration, use 0.9% Sodium Chloride Injection, USP.
3 DOSAGE FORMS AND STRENGTHS
NOVOSEVEN RT is available as a white lyophilized powder in single-dose vials containing 1 mg (1000 micrograms), 2 mg (2000 micrograms), 5 mg (5000 micrograms), or 8 mg (8000 micrograms) recombinant coagulation Factor VIIa (rFVIIa) per vial.
The diluent for reconstitution of NOVOSEVEN RT is a 10 mmol solution of L-histidine in water for injection. It is a clear colorless solution provided in a vial or a pre-filled diluent syringe and is referred to as the histidine diluent.
After reconstitution with the histidine diluent, the final solution contains approximately 1 mg per mL NOVOSEVEN RT (1000 micrograms per mL).
5 WARNINGS AND PRECAUTIONS
5.1 Thrombosis
- •
- Serious arterial and venous thrombotic events have been reported in clinical trials and postmarketing surveillance.
- •
- Patients with congenital hemophilia receiving concomitant treatment with aPCCs (activated prothrombin complex concentrates), older patients particularly with acquired hemophilia and receiving other hemostatic agents, or patients with a history of cardiac, vascular disease or predisposed to thrombotic events may have an increased risk of developing thrombotic events [See Adverse Reactions (6.1) and Drug Interactions (7)].
- •
- Monitor patients who receive NOVOSEVEN RT for development of signs or symptoms of activation of the coagulation system or thrombosis. When there is laboratory confirmation of intravascular coagulation or presence of clinical thrombosis, reduce the dose of NOVOSEVEN RT or stop the treatment, depending on the patient's condition.
5.2 Hypersensitivity Reactions
Hypersensitivity reactions, including anaphylaxis, can occur with NOVOSEVEN RT. Patients with a known hypersensitivity to mouse, hamster, or bovine proteins may be at a higher risk of hypersensitivity reactions. Discontinue infusion and administer appropriate treatment when hypersensitivity reactions occur.
5.3 Antibody Formation in Factor VII Deficient Patients
Factor VII deficient patients should be monitored for prothrombin time (PT) and factor VII coagulant activity before and after administration of NOVOSEVEN RT. If the factor VIIa activity fails to reach the expected level, or prothrombin time is not corrected, or bleeding is not controlled after treatment with the recommended doses, antibody formation may be suspected and analysis for antibodies should be performed.
5.4 Laboratory Tests
Laboratory coagulation parameters (PT/INR, aPTT, FVII:C) have shown no direct correlation to achieving hemostasis. Assays of prothrombin time (PT/INR), activated partial thromboplastin time (aPTT), and plasma FVII clotting activity (FVII:C), may give different results with different reagents. Treatment with NOVOSEVEN has been shown to produce the following characteristics:
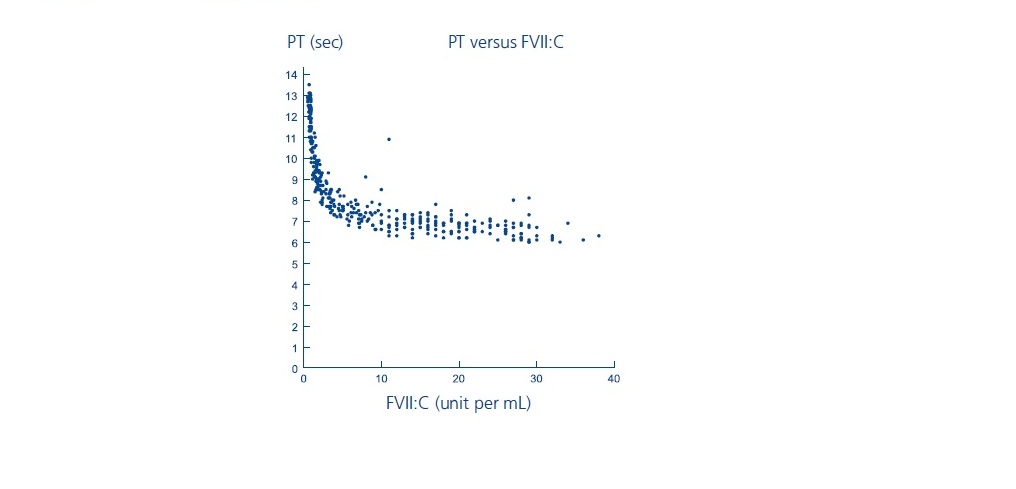
- PT: As shown below, in patients with hemophilia A/B with inhibitors, the PT shortened to about a 7-second plateau at a FVII:C level of approximately 5 units per mL. For FVII:C levels > 5 units per mL, there is no further change in PT. The clinical relevance of prothrombin time shortening following NOVOSEVEN RT administration is unknown.
- INR: NOVOSEVEN has demonstrated the ability to normalize INR. However, INR values have not been shown to directly predict bleeding outcomes, nor has it been possible to demonstrate the impact of NOVOSEVEN on bleeding times/volume in models of clinically-induced bleeding in healthy volunteers who had received Warfarin, when laboratory parameters (PT/INR, aPTT, thromboelastogram) have normalized.
- aPTT: While administration of NOVOSEVEN shortens the prolonged aPTT in hemophilia A/B patients with inhibitors, normalization has usually not been observed in doses shown to induce clinical improvement. Data indicate that clinical improvement was associated with a shortening of aPTT of 15 to 20 seconds.
FVIIa:C: FVIIa:C levels were measured two hours after NOVOSEVEN administration of 35 micrograms per kg body weight and 90 micrograms per kg body weight following two days of dosing at two hour intervals. Average steady state levels were 11 and 28 units per mL for the two dose levels, respectively.
6 ADVERSE REACTIONS
The most common and serious adverse reactions in clinical trials are thrombotic events. Thrombotic adverse reactions following the administration of NOVOSEVEN in clinical trials occurred in 4% of patients with acquired hemophilia and 0.2% of bleeding episodes in patients with congenital hemophilia.
6.1 Clinical Trials Experience
Because clinical studies are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug product cannot be directly compared to rates in clinical trials of another drug, and may not reflect rates observed in practice.
Adverse reactions outlined below have been reported from clinical trials and data collected in registries.
Hemophilia A or B Patients with Inhibitors
In two studies for hemophilia A or B patients with inhibitors treated for bleeding episodes (N=298), adverse reactions were reported in ≥2% of the patients that were treated with NOVOSEVEN for 1,939 bleeding episodes (see Table 3 below).
Table 3: Adverse Reactions Reported in ≥2% of the 298 Patients with Hemophilia A or B with Inhibitors
|
Body System Reactions |
# of adverse reactions (n=1,939 treatments) |
# of patients (n=298 patients) |
|
Body as a whole |
||
|
Fever |
16 |
13 |
|
Platelets, Bleeding, and Clotting |
||
|
Fibrinogen plasma decreased |
10 |
5 |
|
Cardiovascular |
||
|
Hypertension |
9 |
6 |
Serious adverse reactions included thrombosis, pain, thrombophlebitis deep, pulmonary embolism, decreased therapeutic response, cerebrovascular disorder, angina pectoris, DIC, anaphylactic shock and abnormal hepatic function. The serious adverse reactions of DIC and therapeutic response decreased had a fatal outcome.
In two clinical trials evaluating safety and efficacy of NOVOSEVEN administration in the peri-operative setting in hemophilia A or B patients with inhibitors (N=51), the following serious adverse reactions were reported: acute post-operative hemarthrosis (n=1), internal jugular thrombosis adverse reaction (n=1), decreased therapeutic response (n=4).
Immunogenicity
There have been no confirmed reports of inhibitory antibodies against NOVOSEVEN or FVII in patients with congenital hemophilia A or B with alloantibodies.
The incidence of antibody formation is dependent on the sensitivity and specificity of the assay. Additionally, the observed incidence of antibody (including neutralizing antibody) positivity in an assay may be influenced by several factors including assay methodology, sample handling, timing of sample collection, concomitant medications, and underlying disease. For these reasons, comparison of the incidence of antibodies to NOVOSEVEN RT with the incidence of antibodies to other products may be misleading.
Congenital Factor VII Deficiency
Data collected from the compassionate/emergency use programs, the published literature, a pharmacokinetics study, and the Hemophilia and Thrombosis Research Society2 (HTRS) registry showed that 75 patients with Factor VII deficiency had received NOVOSEVEN: 70 patients for 124 bleeding episodes, surgeries, or prophylaxis; 5 patients in the pharmacokinetics trial. The following adverse reactions were reported: intracranial hypertension (n=1), IgG antibody against rFVIIa and FVII (n=1), localized phlebitis (n=1).
Immunogenicity
In 75 patients with factor FVII deficiency treated with NOVOSEVEN RT, one patient developed IgG antibody against rFVIIa and FVII. Patients with factor VII deficiency treated with NOVOSEVEN RT should be monitored for factor VII antibodies.
The incidence of antibody formation is dependent on the sensitivity and specificity of the assay. Additionally, the observed incidence of antibody (including neutralizing antibody) positivity in an assay may be influenced by several factors including assay methodology, sample handling, timing of sample collection, concomitant medications, and underlying disease. For these reasons, comparison of the incidence of antibodies to NOVOSEVEN RT with the incidence of antibodies to other products may be misleading.
Acquired Hemophilia
Data collected from four compassionate use programs, the HTRS registry, and the published literature showed that 139 patients with acquired hemophilia received NOVOSEVEN for 204 bleeding episodes, surgeries and traumatic injuries. Of these 139 patients, 6 patients experienced 8 serious adverse reactions. Serious adverse reactions included shock (n=1), cerebrovascular accident (n=1) and thromboembolic events (n=6) which included cerebral artery occlusion, cerebral ischemia, angina pectoris, myocardial infarction, pulmonary embolism and deep vein thrombosis. Three of the serious adverse reactions had a fatal outcome.
Glanzmann’s Thrombasthenia
Data collected from the Glanzmann’s Thrombasthenia Registry (GTR) and the HTRS registry showed that 140 patients with Glanzmann’s thrombasthenia received NOVOSEVEN RT for 518 bleeding episodes, surgeries or traumatic injuries. The following adverse reactions were reported: deep vein thrombosis (n=1), headache (n=2), fever (n=2), nausea (n=1), and dyspnea (n=1).
7 DRUG INTERACTIONS
- •
- Avoid simultaneous use of activated prothrombin complex concentrates.
- •
- Do not mix NOVOSEVEN RT with infusion solutions.
- •
- Thrombosis may occur if NOVOSEVEN RT is administered concomitantly with Coagulation Factor XIII. [See Warnings and Precautions (5.1) and Nonclinical Toxicology (13.2)]
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Risk Summary
There are no adequate and well-controlled studies using NOVOSEVEN RT in pregnant women to determine whether there is a drug-associated risk.
Treatment of rats and rabbits with NOVOSEVEN in reproduction studies has been associated with mortality at doses up to 6 mg per kg body weight and 5 mg per kg body weight respectively. At 6 mg per kg body weight in rats, the abortion rate was 0 out of 25 litters; in rabbits at 5 mg per kg body weight, the abortion rate was 2 out of 25 litters. Twenty-three out of 25 female rats given 6 mg per kg body weight of NOVOSEVEN gave birth successfully, however, two of the 23 litters died during the early period of lactation. No evidence of teratogenicity was observed after dosing with NOVOSEVEN.
In the U.S. general population, the estimated background risk of major birth defect and miscarriage in clinically recognized pregnancies is 2-4% and 15-20%, respectively.
8.2 Lactation
Risk Summary
There is no information regarding the presence of NOVOSEVEN RT in human milk, the effect on the breastfed infant, and the effects on milk production. The developmental and health benefits of breastfeeding should be considered along with the mother’s clinical need for NOVOSEVEN RT and any potential adverse effects on the breastfed infant from NOVOSEVEN RT or from the underlying maternal condition.
8.4 Pediatric Use
Clinical trials enrolling pediatric patients were conducted with dosing determined according to body weight and not according to age.
Hemophilia A or B with Inhibitors
During the investigational phase of product development NOVOSEVEN was used in 16 children aged 0 to <2 years for 151 bleeding episodes, 27 children aged 2 to <6 years for 140 bleeding episodes, 43 children aged 6 to <12 for 375 bleeding episodes and 30 children aged 12 to 16 years for 446 bleeding episodes.
In a double-blind, randomized comparison trial of two dose levels of NOVOSEVEN in the treatment of joint, muscle and mucocutaneous hemorrhages in hemophilia A and B patients with and without inhibitors 20 children aged 0 to <12 and 8 children aged 12 to 16 were treated with NOVOSEVEN in doses of 35 or 70 micrograms per kg dose. Treatment was assessed as effective (definite relief of pain/tenderness as reported by the patient and/or a measurable decrease of the size of the hemorrhage and/or arrest of bleeding within 8 hours [rated as excellent = 51%], within 8-14 hours [rated as effective = 18%] or after 14 hours [rated as partially effective = 25%]) in 94% of the patients.
NOVOSEVEN was used in two trials in surgery. In a dose comparison 22 children aged 0 to 16 years were treated with NOVOSEVEN. Effective intraoperative hemostasis (defined as bleeding that had stopped completely or had decreased substantially [rated as effective = 86%] or bleeding that was reduced but continued [rated as partially effective = 9%]) was achieved in 21/22 (95%) patients. Effective hemostasis was achieved in 10/10 (100%) patients in the 90 mcg/kg dose group and 10/12 (83%) in the 35 mcg/kg dose group at 48 hours; effective hemostasis was achieved in 10/10 (100%) in the 90 mcg/kg dose group and 9/12 (75%) in the 35 mcg/kg dose group at 5 days.
In the surgery trial comparing bolus (BI) and continuous infusion (CI) 6 children aged 10 to 15 years participated, 3 in each group. Both regimens were 100% effective (defined as bleeding has stopped completely, or decreased substantially) intra-operatively, through the first 24 hours and at day 5. At the end of the study period (Postoperative day 10 or discontinuation of therapy) hemostasis in two patients in the BI group was rated effective and hemostasis in one patient was rated as ineffective (defined as bleeding is the same or has worsened). Hemostasis in all three patients in the CI group was rated as effective.
Adverse drug reactions in pediatric patients were similar to those previously reported in clinical trials with NOVOSEVEN, including one thrombotic event in a 4 year old with internal jugular vein thrombosis after port-a-cath placement which resolved.
Congenital Factor VII deficiency
In published literature, compassionate use trials and registries on use of NOVOSEVEN in congenital Factor VII deficiency, NOVOSEVEN was used in 24 children aged 0 to <12 years and 7 children aged 12 to 16 years for 38 bleeding episodes, 16 surgeries and 8 prophylaxis regimens. Treatment was effective in 95% of bleeding episodes (5% not rated) and 100% of surgeries. No thrombotic events were reported. A seven-month old exposed to NOVOSEVEN and various plasma products developed antibodies against FVII and rFVIIa [see Adverse Reactions (6.1) and Overdosage (10)].
Glanzmann’s Thrombasthenia
In the Glanzmann’s Thrombasthenia Registry, NOVOSEVEN was used in 43 children aged 0 to 12 years for 157 bleeding episodes and in 15 children aged 0 to 12 years for 19 surgical procedures. NOVOSEVEN was also used in 8 children aged >12 to 16 years for 17 bleeding episodes and in 3 children aged >12 to 16 years for 3 surgical procedures. Efficacy of regimens including NOVOSEVEN was evaluated by independent adjudicators as 93.6% and 100% for bleeding episodes in children aged 0 to 12 years and >12 to 16 years, respectively. Efficacy in surgical procedures was evaluated as 100% for all surgical procedures in children aged 0 to 16 years. No adverse reactions were reported in Glanzmann’s thrombasthenia children.
[See Clinical Studies (14)]
10 OVERDOSAGE
Dose limiting toxicities of NOVOSEVEN RT have not been investigated in clinical trials. The following are examples of accidental overdose.
- •
- One newborn female with congenital factor VII deficiency was administered an overdose of NOVOSEVEN (single dose: 800 micrograms per kg body weight). Following additional administration of NOVOSEVEN and various plasma products, antibodies against rFVIIa were detected, but no thrombotic complications were reported.
- •
- One Factor VII deficient male (83 years of age, 111.1 kg) received two doses of 324 micrograms per kg body weight (10-20 times the recommended dose) and experienced a thrombotic event (occipital stroke).
- •
- One hemophilia B patient (16 years of age, 68 kg) received a single dose of 352 micrograms per kg body weight and one hemophilia A patient (2 years of age, 14.6 kg) received doses ranging from 246 micrograms per kg body weight to 986 micrograms per kg body weight on five consecutive days. There were no reported complications in either case.
11 DESCRIPTION
NOVOSEVEN RT, Coagulation Factor VIIa (Recombinant) is a sterile, white lyophilized powder of recombinant human coagulation factor VIIa (rFVIIa) for reconstitution for intravenous injection. The product is supplied as single‑dose vials containing the following:
|
Contents |
1 mg Vial |
2 mg Vial |
5 mg Vial |
8 mg Vial |
|
rFVIIa |
1000 micrograms |
2000 micrograms |
5000 micrograms |
8000 micrograms |
|
sodium chloride* |
2.34 mg |
4.68 mg |
11.7 mg |
18.72 mg |
|
calcium chloride dihydrate* |
1.47 mg |
2.94 mg |
7.35 mg |
11.76 mg |
|
Glycylglycine |
1.32 mg |
2.64 mg |
6.60 mg |
10.56 mg |
|
polysorbate 80 |
0.07 mg |
0.14 mg |
0.35 mg |
0.56 mg |
|
Mannitol |
25 mg |
50 mg |
125 mg |
200 mg |
|
Sucrose |
10 mg |
20 mg |
50 mg |
80 mg |
|
Methionine |
0.5 mg |
1.0 mg |
2.5 mg |
4 mg |
|
*per mg of rFVIIa: 0.4 mEq sodium, 0.01 mEq calcium |
||||
NOVOSEVEN RT also contains trace amounts of proteins derived from the manufacturing and purification processes such as mouse IgG (maximum of 1.2 ng/mg), bovine IgG (maximum of 30 ng/mg), and protein from BHK-cells and media (maximum of 19 ng/mg).
The diluent for reconstitution of NOVOSEVEN RT is a 10 mmol solution of histidine in water for injection and is supplied as a clear colorless solution in a vial or pre-filled diluent syringe. After reconstitution with the appropriate volume of histidine diluent, each vial contains approximately 1 mg/mL NOVOSEVEN RT (corresponding to 1000 micrograms/mL). The reconstituted solution is a clear colorless solution with a pH of approximately 6.0 and contains no preservatives.
Recombinant coagulation Factor VIIa (rFVIIa), the active ingredient in NOVOSEVEN RT, is a vitamin K‑dependent glycoprotein consisting of 406 amino acid residues with an approximate molecular mass of 50 kDa). It is structurally similar to endogenous human coagulation factor VIIa.
The gene for human coagulation factor VII (FVII) is cloned and expressed in baby hamster kidney cells (BHK cells). Recombinant FVII is secreted into the culture media (containing newborn calf serum) in its single-chain form and then proteolytically converted by autocatalysis to the active two-chain form, rFVIIa, during a chromatographic purification process. The purification process has been demonstrated to remove exogenous viruses (MuLV, SV40, Pox virus, Reovirus, BEV, IBR virus). No human serum or other proteins are used in the production or formulation of NOVOSEVEN RT.
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
NOVOSEVEN RT is recombinant Factor VIIa and, when complexed with tissue factor can activate coagulation Factor X to Factor Xa, as well as coagulation Factor IX to Factor IXa. Factor Xa, in complex with other factors, then converts prothrombin to thrombin, which leads to the formation of a hemostatic plug by converting fibrinogen to fibrin and thereby inducing local hemostasis. This process may also occur on the surface of activated platelets.
12.2 Pharmacodynamics
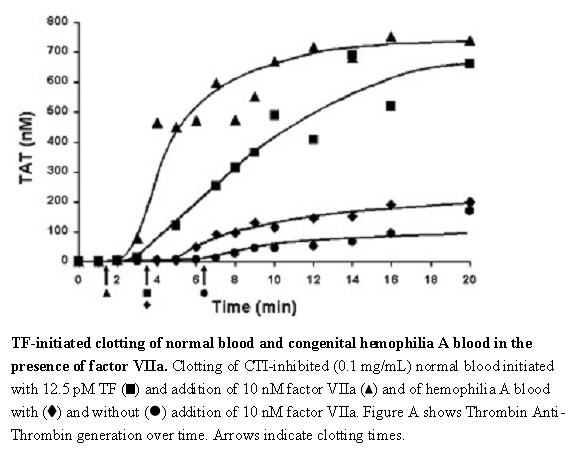
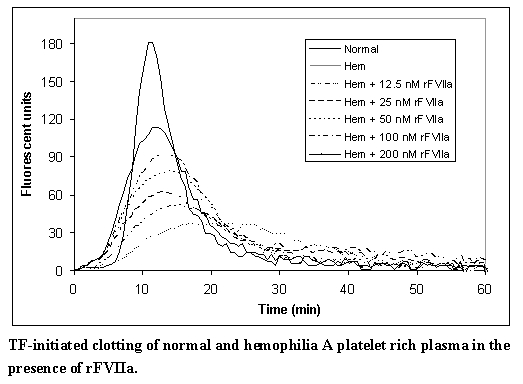
The effect of NOVOSEVEN RT upon coagulation in patients with or without hemophilia has been assessed in different model systems. In an in vitro model of tissue-factor-initiated blood coagulation (Figure A),3 the addition of rFVIIa increased both the rate and level of thrombin generation in normal and hemophilia A blood, with an effect shown at rFVIIa concentrations as low as 10 nM. In this model, fresh human blood was treated with corn trypsin inhibitor (CTI) to block the contact pathway of blood coagulation. Tissue factor (TF) was added to initiate clotting in the presence and absence of rFVIIa for both types of blood.
In a separate model, and in line with previous reports,4 escalating doses of rFVIIa in hemophilia plasma demonstrate a dose-dependent increase in thrombin generation (Figure B). In this model, platelet rich normal and hemophilia plasma was adjusted with autologous plasma to 200,000 platelets/microliter. Coagulation was initiated by addition of tissue factor and CaCl2. Thrombin generation was measured in the presence of a thrombin substrate and various added concentrations of rFVIIa.
Figure A
Figure B
12.3 Pharmacokinetics
Healthy Subjects
The pharmacokinetics of NOVOSEVEN was investigated in 35 healthy subjects (17 Caucasian, 18 Japanese, 16 men, 19 women) in a dose-escalation study. Subjects were dosed with 40, 80 and 160 micrograms per kg NOVOSEVEN.5 No effect of gender or ethnicity on the pharmacokinetics of NOVOSEVEN was observed. Range of mean PK parameters across dose groups are shown in Table 4.
The products NOVOSEVEN® and NOVOSEVEN® RT were found to be pharmacokinetically equivalent in a study of 22 patients receiving single doses of both formulations.6 Mean PK parameters for NOVOSEVEN® RT are shown in Table 4.
Hemophilia A or B
Single dose pharmacokinetics of NOVOSEVEN (17.5, 35, and 70 micrograms per kg) was studied in 15 subjects with hemophilia A or B in non-bleeding and bleeding state.7 Median PK parameters (non-bleeding state) are shown in Table 4.
In a bolus single-dose pharmacokinetic study, 6 male adults (90 micrograms per kg) and 12 male pediatric (2-12 years) patients (crossover, 90 and 180 micrograms per kg) with severe hemophilia A (10 of 18 subjects had inhibitors to factor VIII) received NOVOSEVEN.8 Compared with the adults, body weight normalized clearance of NOVOSEVEN in children 2-5 years and 6-12 years was higher by 82% and 42%, respectively. Pharmacokinetic parameters for children with Hemophilia are shown in Table 5.
Congenital Factor VII deficiency
Single dose pharmacokinetics of NOVOSEVEN in 5 patients with severe congenital Factor VII deficiency (<1%), at doses of 15 and 30 micrograms per kg body weight, showed no significant difference between the two doses. Mean PK parameters for the two doses are shown in Table 4.
Table 4: Single Dose Pharmacokinetic Parameters in Healthy Subjects, Patients With Hemophilia A and B, and Patients With FVII Deficiency (Mean (SD))
|
Healthy Subjects |
Hemophilia A or B |
FVII Deficiency9 |
|||
|
Formulation |
rFVIIa |
rFVIIa-25C |
rFVIIa |
rFVIIa |
rFVIIa |
|
Ages |
20-45 |
22-44 |
15-63 |
30-45 |
20-43 |
|
Doses (mcg/kg) |
40, 80, 160 |
90 |
17.5, 35, 70 |
90 |
30 |
|
AUC |
71.46, 76.91* |
113.26 (17.36)d |
53.31 (20.27)** |
2.45 (0.73) |
23.70 (7.23)d |
|
CL (mL/h) |
1953-2516 |
3077 (438) |
NA |
2767 (385) |
NA |
|
CL (mL/h/kg) |
33-37 |
40.43 (6.23) |
33.84 (11.72) |
37.6 (13.1) |
67.7 (17.9) |
|
t½ (h) |
3.9-6.0 |
3.54 (0.28) |
2.72 (0.54) |
3.2 (0.3) |
2.62 (0.63) |
|
Vss (mL/kg) |
130-165 |
122.96 (20.42) |
108.86 (37.15) |
121 (30) |
230 (70) |
|
MRT (h) |
3.66-4.98 |
3.05 (0.27) |
3.33 (0.64) |
3.31 (0.38) |
3.46 (0.64) |
|
IR ([U/dL]/[U/kg]) |
0.89-1.04 |
1.18 (0.16)c |
NA |
0.94 (0.16) |
0.53 (0.2)c |
- *Based on the 80 mcg/kg dose
** Based on the 70 mcg/kg dose
NA: Not available
AUC: Area under the curve from time 0 to infinity; CL: Clearance; t ½: terminal half-life; Vss: Volume of distribution at steady-state; MRT: mean residence time; IR: Incremental recovery; rFVIIa: NOVOSEVEN® original formulation; rFVIIa-25C: NOVOSEVEN® RT
a: Results are for both males and females. The column presents ranges over ethnicity and gender.
b: Study demonstrated bioequivalence of rFVIIa and rFVIIa-25C (NOVOSEVEN® RT) - c: FVIIa assay used: units in IU
- d: AUC in h*IU/mL
- e: Includes patients with and without inhibitors
Table 5: Single Dose Pharmacokinetic Parameters in Hemophilia A Patients With and Without Inhibitors (Mean (SD))
|
Hemophilia A |
||||||
|
Formulation/ inhibitor status/ age group |
rFVIIa, without inhibitors, age ≤5 years (n=3)8 |
rFVIIa, with inhibitors age ≤5 years (n=2)8 |
rFVIIa, without inhibitors age 6-12 years (n=3)8 |
rFVIIa, with inhibitors age 6-12 years (n=4)8 |
rFVIIa, without inhibitors Adults (n=2)8 |
rFVIIa, with inhibitors Adults (n=4)8 |
|
Ages (years) |
2-5 |
4 |
7-10 |
7-12 |
30-32 |
32-45 |
|
Weight (kg) |
14-17 |
22-26 |
25-38 |
25-68 |
72-97 |
54-89 |
|
Doses (mcg/kg) |
90, 180 |
90, 180 |
90, 180 |
90, 180 |
90 |
90 |
|
AUC (h*U/ mL) |
1.26 (0.09)* |
1.51 (0.25)* |
1.68 (0.24)* |
1.64 (0.31)* |
2.92 (0.80) |
2.13 (0.62) |
|
CL (mL/h) |
1131 (114) |
1387 (75) |
1878 (499) |
1668 (510) |
2477 (162) |
2960 (378) |
|
CL (mL/h/kg) |
73 (8) |
61 (9) |
55 (7) |
52 (12) |
30 (8) |
43 (15) |
|
t½ (h) |
2.6 (0.9) |
1.9 (0.6) |
3.0 (1.1) |
3.0 (0.5) |
3.3 (0.3) |
3.2 (0.3) |
|
Vss (mL/kg) |
191 (44) |
145 (1) |
173 (39) |
149 (22) |
108 (18) |
130 (37) |
|
MRT (h) |
2.6 (0.5) |
2.4 (0.4) |
3.1 (0.5) |
2.9 (0.3) |
3.62 (0.40) |
3.09 (0.20) |
|
IR ([IU/dL]/[U/kg]) |
0.59 (0.06) |
0.75 (0.12) |
0.75 (0.35) |
0.76 (0.20) |
1.01 (0.08) |
0.89 (0.20) |
- *Based upon the 90 mcg/kg dose
- AUC: Area under the curve from time 0 to infinity; CL: Clearance; t ½: terminal half-life; Vss: Volume of distribution at steady state; MRT: mean residence time; IR: Incremental recovery; rFVIIa: (NOVOSEVEN® original formulation)
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
Two mutagenicity studies have given no indication of carcinogenic potential for NOVOSEVEN. The clastogenic activity of NOVOSEVEN was evaluated in both in vitro studies (i.e., cultured human lymphocytes) and in vivo studies (i.e., mouse micronucleus test). Neither of these studies indicated clastogenic activity of NOVOSEVEN. Other gene mutation studies have not been performed with NOVOSEVEN RT (e.g., Ames test). No chronic carcinogenicity studies have been performed with NOVOSEVEN RT.
A reproductive study in male and female rats at dose levels up to 3.0 mg per kg per day had no effect on mating performance, fertility, or litter characteristics.
Treatment of rats and rabbits with NOVOSEVEN in reproduction studies has been associated with mortality at doses up to 6 mg per kg and 5 mg per kg. At 6 mg per kg in rats, the abortion rate was 0 out of 25 litters; in rabbits at 5 mg per kg, the abortion rate was 2 out of 25 litters. Twenty-three out of 25 female rats given 6 mg per kg of NOVOSEVEN gave birth successfully, however, two of the 23 litters died during the early period of lactation. No evidence of teratogenicity was observed after dosing with NOVOSEVEN.
13.2 Animal Toxicology and/or Pharmacology
In a monkey cardiovascular safety pharmacology model evaluating the combination of excessive doses of Coagulation Factor XIII A-Subunit (Recombinant) (585 IU/kg, 17 times the expected human dose) in combination with rFVIIa (1000 mcg/kg, 11 times the expected human dose), one of the twelve monkeys died 4 hours after treatment due to thrombosis. Procoagulant risk factors, including 6 indwelling catheters per monkey and the induction of anesthesia, may have complicated the study results. It is unclear whether the mortality was related to the overdose of one or both products, or a specific interaction between them. Nonclinical and clinical studies with the combination of rFXIII and NOVOSEVEN RT at recommended human doses have not been performed.
14 CLINICAL STUDIES
14.1 Hemophilia A or B with Inhibitors
The largest number of patients (N=483) who received NOVOSEVEN during the investigational phase of product development were in an open protocol study 10,11,12 that began enrollment in 1988, shortly after the completion of the pharmacokinetic study. These patients included persons with hemophilia types A or B (with or without inhibitors), persons with acquired inhibitors to Factor VIII or Factor IX, and a few FVII deficient patients. The clinical situations were diverse and included muscle/joint bleeds, mucocutaneous bleeds, surgical prophylaxis, intracerebral bleeds, and other emergent situations.
A double-blind, randomized comparison trial13 of two dose levels of NOVOSEVEN in the treatment of joint, muscle and mucocutaneous hemorrhages in hemophilia A and B patients with and without inhibitors was conducted in 78 patients who received NOVOSEVEN in treatment centers within 4 to 18 hours after experiencing a bleed. Thirty-five patients were treated at the 35 micrograms per kg dose (59 joint, 15 muscle and 5 mucocutaneous bleeding episodes) and 43 patients were treated at the 70 micrograms per kg dose (85 joint and 14 muscle bleeding episodes). Dosing was repeated at 2.5 hour intervals but ranged up to four hours for some patients. Efficacy was assessed at 12 ± 2 hours or at end of treatment, whichever occurred first. Based on a subjective evaluation by the investigator, the respective efficacy rates for the 35 and 70 micrograms per kg groups were: excellent (definite relief of pain/tenderness as reported by the patient and/or a measurable decrease of the size of the hemorrhage and/or arrest of bleeding within 8 hours) 59% and 60%, effective (definite relief of pain/tenderness as reported by the patient and/or a measurable decrease of the size of the hemorrhage and/or arrest of bleeding within 8-14 hours) 12% and 11%, and partially effective (definite relief of pain/tenderness as reported by the patient and/or a measurable decrease of the size of the hemorrhage and/or arrest of bleeding after 14 hours) 17% and 20%. The average number of injections required to achieve hemostasis was 2.8 and 3.2 for the 35 and 70 micrograms per kg groups, respectively.
Two clinical trials were conducted to evaluate the safety and efficacy of NOVOSEVEN administration during and after surgery in hemophilia A or B patients with inhibitors. One of the studies was a randomized, double-blind, parallel group clinical trial (28 patients with hemophilia A or B and inhibitors and one patient with acquired inhibitor to FVIII, undergoing major or minor surgical procedures).14 Patients received bolus intravenous NOVOSEVEN (either 35 micrograms per kg, N=15; or 90 micrograms per kg, N=14) prior to surgery, intra-operatively as required, then every 2 hours for the following 48 hours beginning at closure of the wound. Additional doses were administered every 2 to 6 hours up to an additional 3 days to maintain hemostasis. After a maximum of 5 days of double-blind treatment, therapy could be continued in an open-label manner if necessary (90 micrograms per kg NOVOSEVEN every 2-6 hours) (Table 6). Efficacy was assessed during the intra-operative period, and post-operatively from the time of wound closure (Hour 0) through Day 5.
Table 6: Dosing by Surgery Category
| Major Surgery | Minor Surgery | |||||||
|---|---|---|---|---|---|---|---|---|
|
||||||||
|
35 μg/kg* (n = 5) |
90 μg/kg (n = 6) |
35 μg/kg (n = 10) |
90 μg/kg (n = 8) |
|||||
|
15 (2-26) |
9.5 (8-17) |
4 (3-6) |
6 (3-13) |
||||
|
135 (11-186) |
81 (71-128) |
29.5 (24-44) |
39.5 (26-98) |
||||
|
656 (31-839) |
569 (107-698) |
45.5 (14-171) |
|
||||
Intraoperative hemostasis was achieved in 28/29 (97%) patients. Satisfactory hemostasis was achieved in 14/14 (100%) patients in the 90 mcg/kg dose group and 11/15 (73%) in the 35 mcg/kg dose group at 48 hours; satisfactory hemostasis was achieved in 13/14 (93%) in the 90 mcg/kg dose group and 11/15 (73%) in the 35 mcg/kg dose group at 5 days. Twenty-three patients successfully completed the entire study including 13/14 (93%) achieving successful hemostasis through study completion (up to day 26) in the 90 mcg/kg dose group.
Another open-label, randomized, parallel trial was conducted to compare the safety and efficacy of bolus intravenous (BI) injection (N=12) and continuous intravenous (CI) infusion (N=12) administration of NOVOSEVEN in 23 hemophilia A or B patients with inhibitors and one patient with acquired hemophilia who were undergoing elective major surgery. Table 7 provides the overview of dosing by treatment group for BI and CI.
Table 7: Dosing by Treatment Group
| Bolus Injection | Continuous Infusion | ||
|---|---|---|---|
|
|
||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
a Includes one patient with acquired hemophilia
b Includes dosing during the follow-up period after the 10-day study period.
Intraoperative hemostasis was reported as effective in all BI and CI treated patients. BI regimen was 100% effective through the first 24 hours, and was 92% effective at day 5. CI regimen was 83% effective through the first 24 hours, and was 83% effective at day 5. At the end of the study period (Post operative day 10 or discontinuation of therapy), hemostatic efficacy in the BI and CI arms was 9/12 (75%) and 10/12 (83%), respectively.
14.2 Congenital Factor VII Deficiency
Data were collected from the published literature, compassionate use trials and registries for 70 patients with Factor VII deficiency treated with NOVOSEVEN for 124 bleeding episodes, surgeries, or prophylaxis regimens. Thirty-two of these patients were enrolled in emergency and compassionate use trials conducted by Novo Nordisk (43 non-surgical bleeding episodes, 26 surgeries); 35 were reported in the published literature (20 surgeries, 10 non-surgical bleeding episodes, 4 cases of caesarean section or vaginal birth, and 10 cases of long-term prophylaxis, and 1 case of on-demand therapy); and 3 were from a registry maintained by the Hemophilia and Thrombosis Research Society (9 bleeding episodes, 1 surgery). Dosing ranged from 6 to 98 micrograms per kg administered every 2-12 hours (except for prophylaxis, where doses were administered from 2 times per day up to 2 times per week). Patients were treated with an average of 1-10 doses. Treatment was effective (bleeding stopped or treatment was rated as effective by the physician) in 93% of episodes (90% for trial patients, 98% for published patients, 90% for HTRS registry patients).
14.3 Acquired Hemophilia
Data were collected from four studies in a compassionate use program conducted by Novo Nordisk and the Hemophilia and Thrombosis Research Society (HTRS) registry. The studies were not designed to select doses or compare first-line efficacy or efficacy when used after failure of other hemostatic agents (salvage treatment). A total of 70 patients with acquired hemophilia were treated with NOVOSEVEN for 113 bleeding episodes, surgeries, or traumatic injuries. Sixty-one of these patients were from the compassionate use program with 100 bleeding episodes (68 non-surgical and 32 surgical bleeding episodes) and 9 patients were from the HTRS registry with 13 bleeding episodes (8 non-surgical, 3 surgical and 2 episodes classified as other). Concomitant use of other hemostatic agents occurred in 29/70 (41%); 13 (19%) received more than one hemostatic agent. The most common hemostatic agents used were antifibrinolytics, Factor VIII and activated prothrombin complex concentrates.
The mean dose of NOVOSEVEN administered was 90 micrograms per kg (range: 31 to 197 micrograms per kg); the mean number of injections per day was 6 (range: 1 to 10 injections per day). Overall efficacy (i.e., effective and partially effective outcomes) was 87/112 (78%); with 77/100 (77%) efficacy in the compassionate use programs and 10/12 (83%) efficacy in the HTRS registry. In the compassionate use programs, overall efficacy for the first-line treatment was 38/44 (86%) compared to 39/56 (70%) when used as salvage treatment (Table 8).
Table 8: Efficacy of NOVOSEVEN in Compassionate Use Programs and HTRS Registry
|
Outcomea |
|
|
Effective |
|
|
Partial |
|
|
Ineffective |
|
|
Unknown |
|
|
No. of Bleeding Episodes |
|
a Outcome assessed at end of treatment, last observation carried forward.
b One patient in the HTRS registry was excluded from efficacy analysis since NOVOSEVEN was used to maintain hemostasis after bleeding had been controlled.
14.4 Glanzmann’s Thrombasthenia
Data were collected from the Glanzmann’s Thrombasthenia Registry (GTR), the Hemostasis and Thrombosis Research Society (HTRS) registry, and the published literature. The GTR was observational, and therefore not designed to select doses. The GTR captured data for 218 Glanzmann’s thrombasthenia patients with 1073 bleeding and surgical events. An independent adjudication committee assessed clinical refractoriness and antibody status based upon historical data from investigators, patterns of treatment, and treatment responses when only platelets were used. Adjudicators defined clinical refractoriness as lack of platelet response. Patients with apparent response to platelets only were not considered refractory, even if coded as such by investigators. Antibody status included GPIIb/IIIa, HLA, and unspecified platelet-specific antibodies. Efficacy was evaluated on a two-point scale (clinical assessment of success or failure of treatment regimen as a whole, blinded and unblinded to investigator coded outcome) in 190 patients with 755 episodes requiring systemic hemostatic therapy (151 patients with 564 severe bleeding episodes, 90 patients with 192 surgeries, one episode was classified as both). Of these a total of 92 patients were treated with NOVOSEVEN RT for 266 bleeding episodes and 77 patients treated for 160 surgical procedures. A large number of bleeding episodes were treated with NOVOSEVEN RT alone (109/266 (41%) events).
The median dose of NOVOSEVEN RT administered for bleeding episodes and surgical procedures was 90 micrograms per kg and the median interval between doses was 3 hours.
For all subjects, concomitant use of other hemostatic agents occurred in 157/266 (59%) bleeding episodes and 94/160 (59%) surgical procedures.
The majority of NOVOSEVEN RT treated bleeding episodes were in pediatric patients (65%; children and adolescents, 0-16 yrs.).
Of the 266 bleeding episodes treated with NOVOSEVEN RT, the most common types of bleeding episodes were: epistaxis (116, 43.6%), gum bleeding (48, 18.0%), menorrhagia (36, 13.5%), tooth/dental extraction related (29, 10.9%), and gastrointestinal (23, 8.6%).
Of the patients treated with NOVOSEVEN RT for surgical procedures, 86% were adults (> 16 years). Major surgery was defined as any invasive operative procedure in which, body cavity was entered, mesenchymal barrier was crossed, facial plane was opened, organ was removed or normal anatomy was operatively altered. Minor surgery was defined as any invasive operative procedure in which only skin, mucous membranes, or superficial connective tissue were manipulated. Surgical procedures treated with NOVOSEVEN RT included minor (134/160; 83.8%) and major (26/160, 16.3%) procedures. Dental procedures were most common (106, 66.3%), followed by endoscopy (12, 7.5%), nasal procedures (8, 5.0%), excision (7, 4.4%), GI surgery (7, 4.4%) and orthopedic procedures (6, 3.8%). Most surgeries were elective (147, 91.9%), with a few emergency (7, 4.4%) or unspecified (6).
Overall, treatment with NOVOSEVEN RT was successful in 94.4% of bleeding episodes (Table 9) and 99.4% of surgical procedures (Table 10). Adjudicator-rated efficacy was consistent across treatment regimens, bleed and surgery types, age, and refractory/antibody status. Treatment with NOVOSEVEN RT was successful in patients with clinical refractoriness with or without platelet-specific antibodies in 94.9% of bleeding episodes and 98.6% of surgical procedures. In patients without refractoriness or platelet-specific antibodies, treatment with NOVOSEVEN RT was comparable to treatment with platelets.
Table 9: Adjudicator Evaluation of Efficacy – Bleeding Episodes for GTR Data
|
No. of patientsc |
No. of episodes |
Success |
Failure |
Insufficient data |
No Consensus | ||
|
All NOVOSEVEN* |
92 |
266 |
251 (94.4) |
4 (1.5) |
6 (2.3) |
5 (1.9) | |
|
By Treatment Regimen | |||||||
|
NOVOSEVEN only |
44 |
109 |
101 (92.7) |
2 (1.8) |
4 (3.7) |
2 (1.8) | |
|
NOVOSEVEN ± Platelets ± Other hemostatic agents |
69 |
157 |
150 (95.5) |
2 (1.3) |
2 (1.3) |
3 (1.9) | |
|
By Antibody/Refractory Group | |||||||
|
Refractoriness ± Platelet-specific antibodiesa,d |
31 |
79 |
75 (94.9) |
2 (2.5) |
2 (2.5) |
0 (0.0) | |
|
Platelet-specific-antibodiesa,d |
8 |
10 |
10 (100.0) |
0 (0.0) |
0 (0.0) |
NA | |
|
Neither or unknownb,d |
57 |
177 |
166 (93.8) |
2 (1.1) |
4 (2.3) |
5 (2.8) | |
*All treatment regimens that included treatment with NOVOSEVEN
a includes GPIIb/IIIa, HLA, and unspecified platelet-specific antibodies
b Assumes no platelet-specific antibodies or refractoriness reported or antibody and refractory status unknown
c Patient numbers are not additive. Patients may have episodes with different treatment regimens and have more than one antibody/refractory status
d Treatment was NOVOSEVEN only for 26/79 episodes with refractoriness with or without antibodies, 2/10 episodes with platelet specific antibodies only, and 81/177 episodes with neither or unknown. The remainder received NOVOSEVEN with platelets and/or antifibrinolytic agents.
Table 10: Adjudicator Evaluation of Efficacy – Surgical Procedures for GTR Data
|
Treatment group |
No. of patientsc |
No. of procedures |
Success |
Insufficient datad |
|
All NOVOSEVEN* |
77 |
160 |
159 (99.4) |
1 (0.6) |
|
By Treatment Regimen |
||||
|
NOVOSEVEN only |
35 |
66 |
65 (98.5) |
1 (1.5) |
|
NOVOSEVEN ± Platelets ± Other hemostatic agents |
57 |
94 |
94 (100.0) |
0 (0.0) |
|
By Antibody/Refractory Group |
||||
|
Refractoriness ± Platelet-specific antibodiesa,e |
33 |
70 |
69 (98.6) |
|
|
Platelet-specific antibodiesa,e |
11 |
24 |
24 (100.0) |
|
|
Neither or unknownb,e |
36 |
66 |
66 (100) |
|
*All treatment regimens that included treatment with NOVOSEVEN
a includes GPIIb/IIIa, HLA, and unspecified platelet-specific antibodies
b Assumes no platelet-specific antibodies or refractoriness reported or antibody and refractory status unknown
c Patient numbers are not additive. Patients may have episodes with different treatment regimens and have more than one antibody/refractory status
d No reports of failure or lack of consensus was reported
e Treatment was NOVOSEVEN only for 22/70 episodes with refractoriness with or without antibodies, 13/24 episodes with platelet specific antibodies only, and 31/66 episodes with neither or unknown. The remainder received NOVOSEVEN with platelets and/or antifibrinolytic agents.
In HTRS, there were 7 patients that were treated with NOVOSEVEN RT for 23 bleeding episodes. Concomitant hemostatic agents were administered for 11 episodes (antifibrinolytics in 10 episodes). Treatment was reported effective in 21 of 23 (91.3%) episodes. In the other 2 episodes, bleeding was reported as slowed or no improvement, however in neither episode was further treatment reported. There were no surgical procedures reported in the HTRS registry.
15 REFERENCES
- 1. Hedner, U.: Dosing and Monitoring NOVOSEVEN ® Treatment, Haemostasis 1996; 26 (suppl 1): 102-108.
- 2. Parameswaran, R., et al.: Dose effect and efficacy of rFVIIa in the treatment of haemophilia patients with inhibitors: analysis from the Hemophilia and Thrombosis Research Society Registry, Haemophilia 2005; 11: 100-106.
- 3.Butenas, S., et al.: Mechanism of factor VIIa-dependent coagulation in hemophilia blood, Blood 2002; 99: 923-930. Figure A Copyright American Society of Hematology, used with permission.
- 4. Allen, G.A., et al.: The effect of factor X level on thrombin generation and the procoagulant effect of activated factor VII in a cell-based model of coagulation, Blood Coagulation and Fibrinolysis 2000; 11 (suppl 1): 3-7.
- 5. Fridberg M.J., et al.: A study of the pharmacokinetics and safety of recombinant activated factor VII in healthy Caucasian and Japanese subjects, Blood Coagulation and Fibrinolysis 2005; 16 (4): 259-266.
- 6. Bysted B.V., et al.: A randomized double-blind trial demonstrating bioequivalence of the current recombinant activated factor VII formulation and a new robust 25ºC stable formulation, Haemophilia 2007; 13, 527-532.
- 7. Lindley, C.M., et al.: Pharmacokinetics and pharmacodynamics of recombinant Factor VIIa, Clinical Pharmacology & Therapeutics 1994; 55 (6): 638-648.
- 8.Villar, A., et al.: Pharmacokinetics of activated recombinant coagulation factor VIIa (NOVOSEVEN®) in children vs. adults with haemophilia A, Haemophilia 2004; 10 (4):352-359.
- 9. Berretinni M.: Pharmacokinetic evaluation of recombinant, activated factor VII in patients with inherited factor VII deficiency. Haematologica 2001; 86:640-645.
- 10. Lusher, J., et al.: Clinical experience with recombinant Factor VIIa, Blood Coagulation and Fibrinolysis 1998; 9: 119-128.
- 11. Bech, M.R.: Recombinant Factor VIIa in Joint and Muscle Bleeding Episodes, Haemostasis 1996; 26 (suppl 1): 135-138.
- 12. Lusher, J.M.: Recombinant Factor VIIa (NOVOSEVEN ® ) in the Treatment of Internal Bleeding in Patients with Factor VIII and IX Inhibitors, Haemostasis 1996; 26 (suppl 1): 124-130.
- 13. Lusher, J.M., et al.: A randomized, double-blind comparison of two dosage levels of recombinant factor VIIa in the treatment of joint, muscle and mucocutaneous haemorrhages in persons with hemophilia A and B, with and without inhibitor, Haemophilia 1998; 4: 790-798.
- 14. Shapiro A.D., et al: Prospective, Randomised Trial of Two Doses of rFVIIa (NOVOSEVEN ® ) in Haemophilia Patients with Inhibitors Undergoing Surgery, Thrombosis and Haemostasis 1998; 80: 773-778.
16 HOW SUPPLIED/STORAGE AND HANDLING
How Supplied
NOVOSEVEN RT, Coagulation Factor VIIa (Recombinant), is supplied as a room temperature stable, white, lyophilized powder in single‑dose vials, one single-dose vial per carton. The diluent for reconstitution of NOVOSEVEN RT is a 10 mmol solution of L-histidine in water for injection and is supplied as a clear colorless solution, and referred to as the histidine diluent. The histidine diluent is provided in either a vial or pre-filled diluent syringe.
The amount of rFVIIa in milligrams and in micrograms is stated on the label.
NOVOSEVEN RT package containing 1 single-dose vial of NOVOSEVEN RT powder and 1 vial of histidine diluent:
|
Presentation |
Carton NDC Number |
Components |
|
1 mg per vial (1000 micrograms/vial) |
NDC 0169 7010 01 |
|
|
2 mg per vial (2000 micrograms/vial) |
NDC 0169 7020 01 |
|
|
5 mg per vial (5000 micrograms/vial) |
NDC 0169 7050 01 |
|
|
8 mg per vial (8000 micrograms/vial) |
NDC 0169 7040 01 |
|
NOVOSEVEN RT with MixPro® package containing 1 single-dose vial of NOVOSEVEN RT powder and 1 pre-filled histidine diluent syringe with sterile vial adapter which serves as an alternative needleless reconstitution system:
|
Presentation |
Carton NDC Number |
Components |
|
1 mg per vial (1000 micrograms/vial) |
NDC 0169 7201 01 |
|
|
2 mg per vial (2000 micrograms/vial) |
NDC 0169 7202 01 |
|
|
5 mg per vial (5000 micrograms/vial) |
NDC 0169 7205 01 |
|
|
8 mg per vial (8000 micrograms/vial) |
NDC 0169 7208 01 |
|
The NOVOSEVEN RT and histidine diluent vials are made of glass closed with a chlorobutyl rubber stopper not made with natural rubber latex, and covered with an aluminum cap. The pre-filled diluent syringes are made of glass, with a siliconised bromobutyl rubber plunger not made with natural rubber latex. The closed vials and pre-filled diluent syringes are equipped with a tamper-evident snap-off cap which is made of polypropylene. A vial adapter with 25 micrometer filter is provided with the pre-filled diluent syringe.
Storage and Handling
Prior to reconstitution, store NOVOSEVEN RT powder and histidine diluent between 2-25°C (36-77°F). Do not freeze. Store protected from light. Do not use past the expiration date.
After reconstitution, store NOVOSEVEN RT either at room temperature or refrigerated for up to 3 hours. Do not freeze reconstituted NOVOSEVEN RT or store in syringes.
17 PATIENT COUNSELING INFORMATION
Advise the patient:
- •
- To read the FDA-approved patient labeling (Instructions for Use).
- •
- About the early signs of hypersensitivity reactions, including hives, urticaria, tightness of the chest, wheezing, hypotension, and anaphylaxis.
- •
- About the signs of thrombosis, including new onset swelling and pain in the limbs or abdomen, new onset chest pain, shortness of breath, loss of sensation or motor power, or altered consciousness or speech.
- •
- To immediately seek medical help if any of the above signs or symptoms occur.
- •
- To follow the recommendations in the FDA-approved patient labeling, regarding proper sharps disposal.
Version: 2020July-V20
PATENT Information: http://novonordisk-us.com/products/product-patents.html
Novo Nordisk® is a registered trademark of Novo Nordisk A/S.
NOVOSEVEN® is a registered trademark of Novo Nordisk Health Care AG.
MixPro® is a registered trademark of Novo Nordisk A/S.
Clave® and MicroClave® are registered trademarks of ICU Medical Inc.
InVision-Plus®, InVision-Plus CS®, InVision-Plus® Junior® are registered trademarks of RyMed Technologies, Inc.
Bionector® is a registered trademark of Vygon.
© 2020 Novo Nordisk
For information contact:
Novo Nordisk Inc.
800 Scudders Mill Road
Plainsboro, NJ 08536, USA
1-877-NOVO-777
Manufactured by:
Novo Nordisk A/S
2880 Bagsvaerd, Denmark
License Number: 1261
Patient Package Insert
FDA Approved Patient Labeling
Instructions for Use
NovoSeven RT
Coagulation Factor VIIa (Recombinant)
Instructions on how to use NovoSeven® RT
READ THESE INSTRUCTIONS CAREFULLY BEFORE USING NOVOSEVEN® RT.
NovoSeven® RT is supplied as a powder. Before injection (administration) it must be mixed (reconstituted) with the liquid diluent supplied in the syringe. The liquid diluent is a histidine solution. The mixed NovoSeven® RT must be injected into your vein (intravenous injection). The equipment in this package is designed to mix and inject NovoSeven® RT.
You will also need an infusion set (tubing and butterfly needle), sterile alcohol swabs, gauze pads, and bandages.
Don’t use the equipment without proper training from your doctor or nurse.
Always use a clean and germ free (aseptic) technique. It is important that you wash your hands and ensure that the area around you is clean.
Don’t open the equipment until you are ready to use it.
The equipment is for single use only.
Single-dose vial. Discard unused portion.
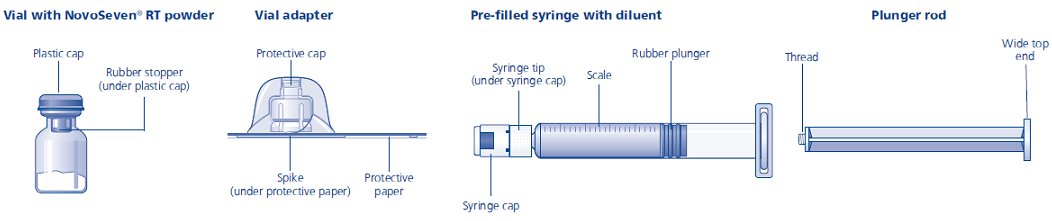
Content
The package contains:
• Vial with NovoSeven® RT powder
• Vial adapter
• Pre-filled syringe with diluent
• Plunger rod (placed under the syringe)
- Overview
1. Prepare the vial and the syringe
- •
- Take out the number of NovoSeven® RT packages you need. Check the expiry date.
- •
- Check the name and the color of the package, to make sure it contains the correct product.
- •
- Wash your hands and dry them properly using a clean towel or air dry.
- •
- Take the vial, the vial adapter and the pre-filled syringe out of the carton. Leave the plunger rod untouched in the carton.
- •
- Bring the vial and the pre-filled syringe to room temperature (not above 98.6°F (37°C)). You can do this by holding them in your hands until they feel as warm as your hands.
- •
- Remove the plastic cap from the vial. If the plastic cap is loose or missing, don’t use the vial.
- •
- Wipe the rubber stopper on the vial with a sterile alcohol swab and allow it to dry for a few seconds before use. Don’t touch the rubber stopper after wiping it.
Don’t use the equipment if it has been dropped, or if it is damaged. Use a new package instead.
Don’t use the equipment if it is expired. Use a new package instead. The expiration date is printed on the outer carton and on the vial, the vial adapter and the pre-filled syringe.
Don’t dispose of any of the items until after you have injected the mixed solution.
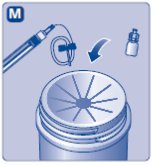
2. Attach the vial adapter
- •
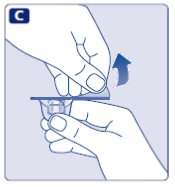
- Remove the protective paper from the vial adapter.
- Don’t take the vial adapter out of the protective cap.
- If the protective paper is not fully sealed or if it is broken, don’t use the vial adapter.
- •
- Place the vial on a flat and solid surface.
- •
- Turn over the protective cap, and snap the vial adapter onto the vial.
- Don’t touch the spike on the vial adapter.
- Once attached, don’t remove the vial adapter from the vial.
- •
- Lightly squeeze the protective cap with your thumb and index finger as shown.
- Remove the protective cap from the vial adapter.
- Don’t lift the vial adapter from the vial when removing the protective cap.
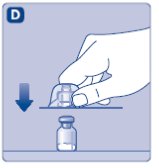
3. Attach the plunger rod and the syringe
- •
- Grasp the plunger rod by the wide top end and take it out of the carton. Be careful not to touch the sides or the thread of the plunger rod. Keep holding the plunger rod at the wide top end.
- •
- Immediately connect the plunger rod to the syringe by turning it clockwise into the rubber plunger inside the pre-filled syringe until resistance is felt.
- •
- Remove the syringe cap from the pre-filled syringe by bending it down until the perforation breaks.
- Don’t touch the syringe tip under the syringe cap.
- If the syringe cap is loose or missing, don’t use the pre-filled syringe.
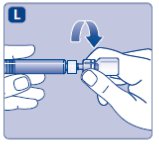
- •
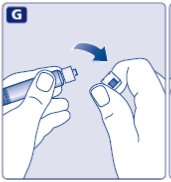
- Screw the pre-filled syringe securely onto the vial adapter until resistance is felt.
Avoid touching the sides of the plunger rod at any time.
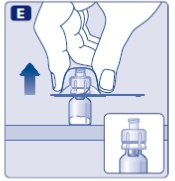
4. Mix the powder with the diluent
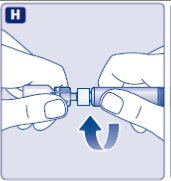
- •
- Hold the pre-filled syringe slightly tilted with the vial pointing downwards.
- •
- Push the plunger rod to inject all the diluent into the vial.
- •
- Keep the plunger rod pressed down and swirl the vial gently until all the powder is dissolved.
- Don’t shake the vial as this will cause foaming.
- •
- Check the mixed solution. It must be clear and colorless. If you notice visible particles or discoloration, don’t use it. Use a new package instead.
NovoSeven® RT is recommended to be used immediately after it is mixed.
If you cannot use the mixed NovoSeven® RT solution immediately, it can be kept in the vial, still with the vial adapter and the syringe attached, at room temperature or refrigerated for no longer than 3 hours.
Do not freeze mixed NovoSeven® RT solution or store it in syringes.
Keep mixed NovoSeven® RT solution out of direct light.
If your dose requires more than one vial, repeat step A to J with additional vials, vial adapters and pre-filled syringes until you have reached your required dose.
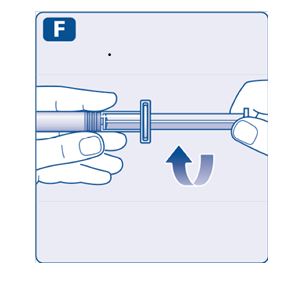
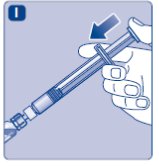
- •
- Keep the plunger rod pushed completely in.
- •
- Turn the syringe with the vial upside down.
- •
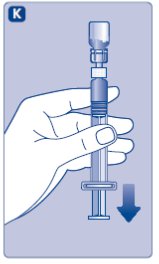
- Stop pushing the plunger rod and let it move back on its own while the mixed solution fills the syringe.
- •
- Pull the plunger rod slightly downwards to draw the mixed solution into the syringe. In case you only need part of the entire dose, use the scale on the syringe to see how much mixed solution you withdraw, as instructed by your doctor or nurse.
- •
- While holding the vial upside down, tap the syringe gently to let any air bubbles rise to the top.
- •
- Push the plunger rod slowly until all air bubbles are gone.
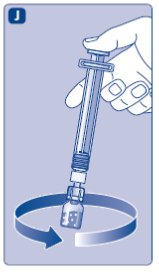
- •
- Unscrew the vial adapter with the vial.
Caution: The pre-filled diluent syringe is made of glass with an internal tip diameter of 0.037 inches, and is compatible with a standard Luer-lock connector.
Some needleless connectors for intravenous catheters are incompatible with the glass diluent syringes (for example, certain connectors with an internal spike, such as Clave® /MicroClave®, InVision-Plus®, InVision-Plus CS®, InVision-Plus® Junior®, Bionector®), and their use can damage the connector and affect administration. To administer product through incompatible needleless connectors, withdraw reconstituted product into a standard 10 mL sterile Luer-lock plastic syringe.
If you have encountered any problems with attaching the pre-filled histidine diluent syringe to any Luer-lock compatible device, please contact Novo Nordisk at (877) 668-6777.
5. Inject the mixed solution
NovoSeven® RT is now ready to inject into your vein.
- •
- Do not mix NovoSeven® RT with any other intravenous infusions or medications.
- •
- Inject the mixed solution slowly over 2 to 5 minutes as instructed by your doctor or nurse.
Injecting the solution via a central venous access device (CVAD) such as a central venous catheter or subcutaneous port:
- •
- Use a clean and germ free (aseptic) technique. Follow the instructions for proper use for your connector and central venous access device in consultation with your doctor or nurse.
- •
- Injecting into a CVAD may require using a sterile 10 mL plastic syringe for withdrawal of the mixed solution and injection.
- •
- If necessary, use 0.9% Sodium Chloride Injection, USP to flush the CVAD line before or after NovoSeven® RT injection.
The peel-off label found on the NovoSeven® RT vial can be used to record the lot number.
Disposal
- •
- After injection, safely dispose of the syringe with the infusion set, the vial with the vial adapter, any unused NovoSeven® RT and other waste materials as instructed by your doctor or nurse.
- Don’t throw it out with the ordinary household trash.
Don’t disassemble the vial and vial adapter before disposal.
Don’t reuse the equipment.
For full Prescribing Information please read the other insert included in this package.
Revised: 07/2020
PATENT information: http://novonordisk-us.com/products/product-patents.html
Novo Nordisk® is a registered trademark of Novo Nordisk A/S.
NovoSeven® is a registered trademark of Novo Nordisk Health Care AG.
Clave® and MicroClave® are registered trademarks of ICU Medical Inc.
InVision-Plus®, InVision-Plus CS®, InVision-Plus® Junior® are registered trademarks of RyMed Technologies, Inc.
Bionector® is a registered trademark of Vygon.
© 2020 Novo Nordisk
Manufactured by:
Novo Nordisk A/S
2880 Bagsvaerd, Denmark
License Number: 1261
Version: 20200710-v8
Principal Display Panel 1 mg
NDC 0169 7010 01
List: 701001
NovoSeven® RT
Coagulation Factor VIIa
(Recombinant)
Room Temperature Stable
1 mg
For IV administration only
For human use only
Single dose vial
Administer within 3 hours of reconstitution
Contains no preservative
Rx only
novo nordisk®
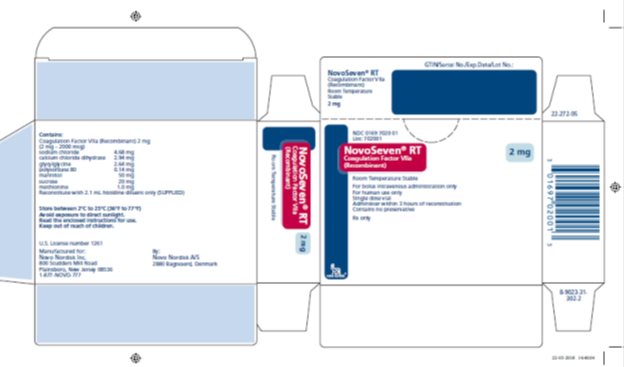
Principal Display Panel 2mg
NDC 0169 7020 01
List: 702001
NovoSeven® RT
Coagulation Factor VIIa
(Recombinant)
Room Temperature Stable
2 mg
For IV administration only
For human use only
Single dose vial
Administer within 3 hours of reconstitution
Contains no preservative
Rx only
novo nordisk®
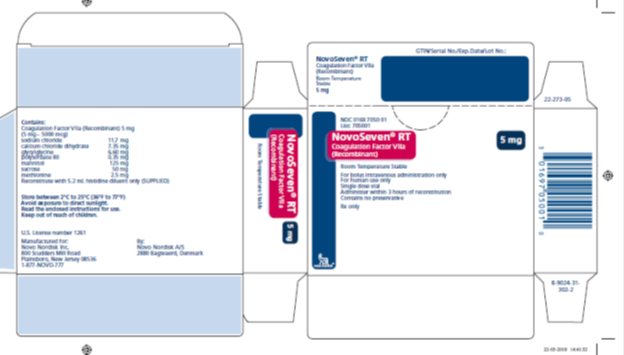
Principal Display Panel 5 mg
NDC 0169 7050 01
List: 705001
NovoSeven® RT
Coagulation Factor VIIa
(Recombinant)
Room Temperature Stable
5 mg
For IV administration only
For human use only
Single dose vial
Administer within 3 hours of reconstitution
Contains no preservative
Rx only
novo nordisk®
Principal Display Panel 8 mg
NDC 0169 7040 01
List: 704001
NovoSeven® RT
Coagulation Factor VIIa
(Recombinant)
Room Temperature Stable
8 mg
For IV administration only
For human use only
Single dose vial
Administer within 3 hours of reconstitution
Contains no preservative
Rx only
novo nordisk®
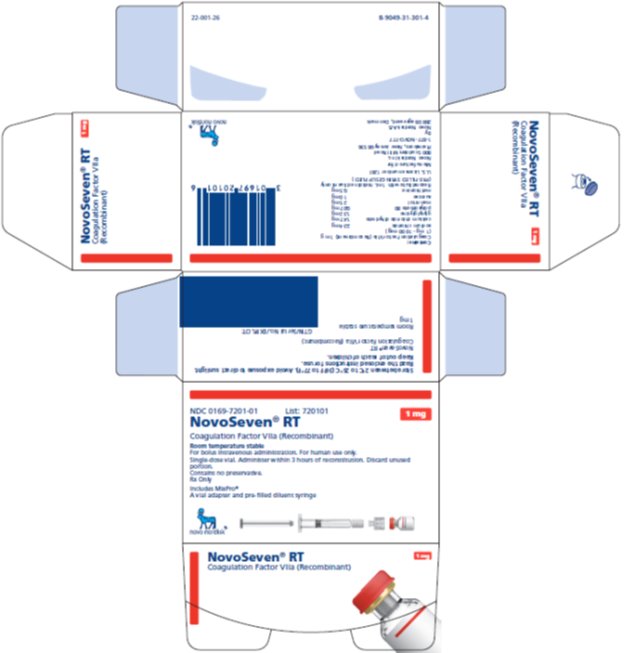
Principal Display Panel 1 mg
NDC 0169-7201-01
- List: 720101 1 mg
- NovoSeven® RT
- Coagulation Factor VIIa (Recombinant)
Room Temperature Stable
For bolus intravenous administration. For human use only.
Single-dose vial. Administer within 3 hours of reconstitution. Discard unused portion.
Contains no preservative.
Rx Only
- Includes MixPro®
- A vial adapter and pre-filled diluent syringe
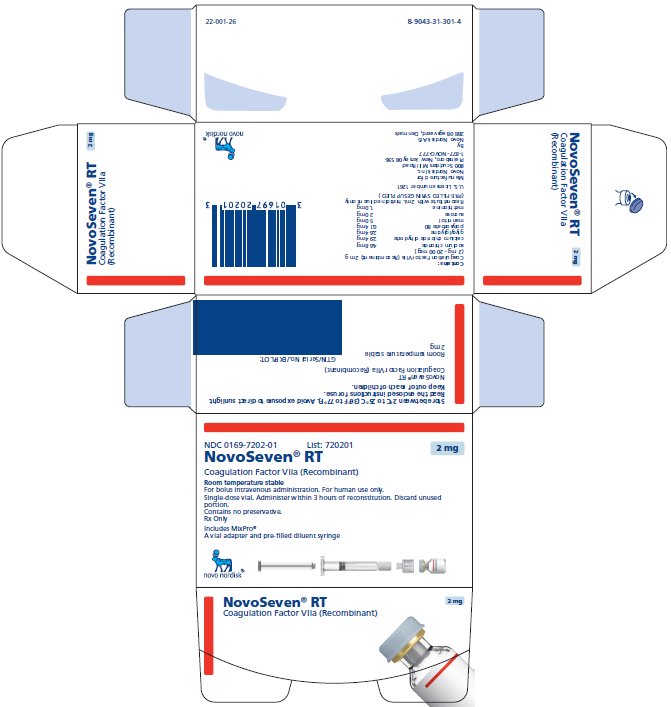
Principal Display Panel 2 mg
NDC 0169-7202-01
- List: 720201 2 mg
- NovoSeven® RT
- Coagulation Factor VIIa (Recombinant)
Room Temperature Stable
For bolus intravenous administration. For human use only.
Single-dose vial. Administer within 3 hours of reconstitution. Discard unused portion.
Contains no preservative.
Rx Only
Includes MixPro®
A vial adapter and pre-filled diluent syringe
Principal Display Panel 5 mg
NDC 0169-7205-01
- List: 720501 5 mg
- NovoSeven® RT
- Coagulation Factor VIIa (Recombinant)
Room Temperature Stable
For bolus intravenous administration. For human use only.
Single-dose vial. Administer within 3 hours of reconstitution. Discard unused portion.
Contains no preservative.
Rx Only
Includes MixPro®
Principal Display Panel 8 mg
NDC 0169-7208-01
- List: 720801 8 mg
- NovoSeven® RT
- Coagulation Factor VIIa (Recombinant)
Room Temperature Stable
For bolus intravenous administration. For human use only.
Single-dose vial. Administer within 3 hours of reconstitution. Discard unused portion.
Contains no preservative.
Rx Only
Includes MixPro®
A vial adapter and pre-filled diluent syringe