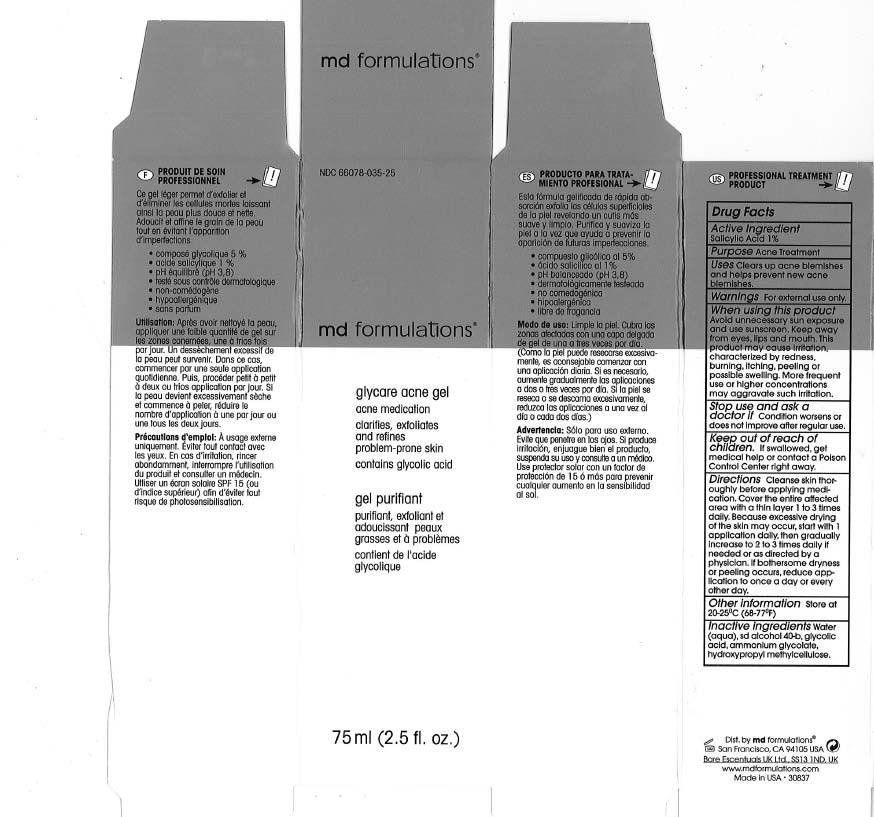
GLYCARE ACNE - glycolic acid gel
MD Formulation
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
If swallowed, get medical help or contact a Poison Control Center right away.
Clears up acne blemishes and helps prevent new acne blemishes.
Directions Cleanse skin thoroughly before applying medication.Cover the entire affected area with a thin layer 1 to 3 times dally. Because excessive drying of the skin may occur, start with 1 application daily, then gradually increase to 2 to 3 times daily if needed or as directed by a physician. If bothersome dryness or peeling occurs, reduce application to once a day or every other day.
Water (aqua), sd alcohol 40-b, glycolic acid, ammonium glycolate, hydroxypropyl melhylcellulose.