FULL PRESCRIBING INFORMATION
2 DOSAGE AND ADMINISTRATION
The recommended dose of IHEEZO TM is 3 drops applied topically to the ocular surface in the area of the planned procedure. IHEEZO TM may be reapplied as needed to maintain anesthetic effect.
3 DOSAGE FORMS AND STRENGTHS
IHEEZO TM (chloroprocaine hydrochloride ophthalmic gel) 3% contains 24 mg of chloroprocaine hydrochloride per vial (800 mg of gel).
4 CONTRAINDICATIONS
IHEEZO TM is contraindicated in patients with a history of hypersensitivity to any component of this preparation.
5 WARNINGS AND PRECAUTIONS
5.1 Not for Injection or Intraocular Administration
IHEEZO TM should not be injected or intraocularly administered.
5.2 Corneal Injury Due to Insensitivity
Patients should not touch the eye for at least 10 to 20 minutes after using anesthetic as accidental injuries can occur due to insensitivity of the eye.
5.3 Corneal Opacification
Prolonged use of a topical ocular anesthetic may produce permanent corneal opacification and ulceration with accompanying visual loss.
6 ADVERSE REACTIONS
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
The data described below reflect 201 patients undergoing various surgical ocular procedures in two placebo-controlled trials (Study 1 and Study 2). Patients in Study 1 were randomized to receive a single instillation of 3 drops of IHEEZO TM or placebo. Patients in Study 2 were randomized to receive a single or multiple instillations of 1, 3 or 3+3 drops of IHEEZO TM or placebo.
The most common adverse reactions in these studies, (incidence greater than or equal to 5%) following IHEEZO TM administration were mydriasis, conjunctival hyperemia and eye irritation.
Adverse Reactions Reported in Controlled Trials
Table 1. Adverse Reactions in 5% or more of IHEEZO TM Treated Patients in Studies 1 and 2
| IHEEZO TM | Placebo | |
| Preferred Term |
N=151 n (%) |
N=50 n (%) |
| Mydriasis | 39 (26%) |
1 (2%) |
| Conjunctival hyperemia | 16 (11%) | 6 (12%) |
| Eye irritation | 9 (6%) | 2 (4%) |
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Risk Summary
There are no adequate and well-controlled studies of IHEEZO TM use in pregnant women to inform a drug associated risk. There are no animal reproduction studies for chloroprocaine.
8.2 Lactation
Risk Summary
There are no data on the presence of chloroprocaine in human milk, the effects on the breastfed infant, or the effects on milk production. The developmental and health benefits of breastfeeding should be considered along with the mother’s clinical need for IHEEZO TM and any potential adverse effects on the breastfed infant from IHEEZO TM.
11 DESCRIPTION
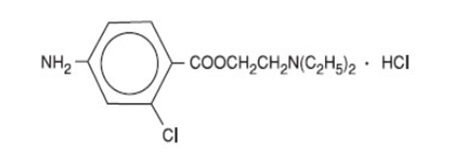
IHEEZO TM is a sterile, single-patient‑use ophthalmic gel preparation for topical ocular anesthesia containing chloroprocaine hydrochloride as the active pharmaceutical ingredient. Chloroprocaine hydrochloride is an ester anesthetic. It is a water-soluble white crystalline powder and its chemical name is 2-(Diethylamino)ethyl 4‑amino-2-chlorobenzoate monohydrochloride. The molecular weight is 307.22 and the molecular formula is C 13H 19ClN 2O 2·HCl. It is represented by the following structural formula:
IHEEZO TM contains:
Active: 30 mg of chloroprocaine hydrochloride (equivalent to 26 mg of chloroprocaine) per gram of gel.
Inactive ingredients: Hydroxyethyl Cellulose (HEC), and Water for Injection. The pH may be adjusted to 3.0 to 5.0 with Hydrochloric Acid. This product does not contain an antimicrobial preservative.
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
12.1 Mechanism of Action
Chloroprocaine, like other local anesthetics, blocks the generation and the conduction of nerve impulses, presumably by increasing the threshold for electrical excitation in the nerve, by slowing the propagation of the nerve impulse, and by reducing the rate of rise of the action potential. In general, the progression of anesthesia is related to the diameter, myelination, and conduction velocity of affected nerve fibers. Clinically, the order of loss of nerve function is as follows: (1) pain, (2) temperature, (3) touch, (4) proprioception, and (5) skeletal muscle tone.
12.3 Pharmacokinetics
The systemic exposure to chloroprocaine following topical ocular administration of IHEEZO TM has not been studied.
Elimination
Metabolism
Chloroprocaine is metabolized by plasma pseudocholinesterases and nonspecific esterases in ocular tissues. Chloroprocaine is rapidly metabolized in plasma by hydrolysis of the ester linkage by pseudocholinesterase. The hydrolysis of chloroprocaine results in the production of ß-diethylaminoethanol and 2-chloro-4-aminobenzoic acid, which inhibits the action of the sulfonamides.
Excretion
Chloroprocaine plasma half-life in vitro is approximately 25 seconds in adults and approximately 43 seconds in neonates. The kidney is the main excretory organ for most local anesthetics and their metabolites. Urinary excretion is affected by urinary perfusion and factors affecting urinary pH.
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
Carcinogenesis
Long-term studies in animals to evaluate carcinogenic potential of chloroprocaine have not been conducted.
Mutagenesis
2-chloroprocaine and the main metabolite, ACBA, were negative in the in vitro bacterial reverse mutation test (Ames assay) and the in vitro chromosome aberrations assay.
Impairment of Fertility
Studies in animals to evaluate the impairment of fertility have not been conducted with chloroprocaine.
14 CLINICAL STUDIES
14.1 Study 1 and 2
Study 1 (NCT04779606) and Study 2 (NCT04753710) were randomized, double-blinded placebo-controlled studies conducted to evaluate the efficacy, safety, and local tolerability of IHEEZO TM in 145 healthy volunteers.
In Study 1, 85 healthy male and female were randomized in a 4:1 ratio to receive a single ocular instillation of IHEEZO TM (N=68) or placebo (N=17). The double blinded treatment included a IHEEZO TM or a placebo dose of 3 drops instilled at 1 minute ± 15 seconds intervals in the right eye of each volunteer. The median age was 39 years (range 19 to 55 years); 59% female and 41% male.
In Study 2, 60 healthy male and female were randomized (40:20) to receive single or multiple ocular instillations of IHEEZO TM dose of 3 drops in the right eye. The median age was 25 years (range 18 to 59 years); 54% female ad 46% male.
The efficacy in Study 1 and 2 was determined by proportion of patients achieving full conjunctival anesthesia evaluated by conjunctival pinching, 5 minutes after administration.
Efficacy results of Study 1
The proportion of subjects with successful anesthesia was 90% in IHEEZO TM group and 12% in the placebo group (p<0.01). The median time for the IHEEZO TM group achieving anesthesia was 0.67 minutes. The median duration of anesthesia was 14.3 minutes.
Efficacy results of Study 2
The proportion of subjects with successful anesthesia was 95% in the IHEEZO TM group and 20% in the placebo group (p<0.01). The median time for the IHEEZO TM group achieving anesthesia was 0.67 minutes. The median duration of anesthesia was 19.3 minutes.
14.2 Study 3
Study 3 (NCT04685538) was a randomized, prospective, multi-center, active-controlled, observer-masked study conducted to evaluate the efficacy and safety of IHEEZO TM (N=166) versus tetracaine ophthalmic solution 0.5% (N=172) in patients undergoing cataract surgery.
The primary endpoint was defined as the proportion of patients in each treatment group gaining successful anesthesia without any supplementation. On average, patients needed 1-1.5 minutes to obtain sufficient anesthesia to successfully perform the surgical procedure which lasted on average 22 minutes.
No patient treated with IHEEZO TM required supplemental treatment to complete the intended surgical procedure.
16 HOW SUPPLIED/STORAGE AND HANDLING
IHEEZO TM (chloroprocaine hydrochloride ophthalmic gel) 3% is supplied as a sterile, clear, colorless to light yellow gel in a single-patient‑use vial. Each single‑patient‑use vial contains 24 mg chloroprocaine in 800 mg of gel.
Aluminum pouch containing 1 LDPE single-patient‑use vial of IHEEZO TM.
The outer surface of the vial is not sterile.
NDC 82667-300-01 Package of 1 unit of 1.25 mL single-patient‑use vial (800 mg filled)
NDC 82667-300-10 Package of 10 units of 1.25 mL single-patient‑use vials (800 mg filled)
NDC 82667-300-00 Sample package of 1 unit of 1.25 mL single-patient-use vial (800 mg filled)
Storage
Store at 15°C to 25°C (59°F to 77°F).
Discard after use.
17 PATIENT COUNSELING INFORMATION
Eye Care Precaution
Do not touch the dropper tip to any surface as this may contaminate the gel.
Advise patients that their eyes will be insensitive for up to 20 minutes due to the effect of the anesthetic, and that care should be taken to avoid accidental injuries.
Manufactured by
Laboratoire Unither
1 Rue de l’Arquerie
50200 COUTANCES
France
Distributed by
Harrow Eye, LLC
102 Woodmont Blvd. Suite 610
Nashville, TN 37205
USA
PRINCIPAL DISPLAY PANEL - Carton
NDC 82667-300-01 Sterile Rx Only
Iheezo TM
(chloroprocaine hydrochloride ophthalmic gel) 3%
For topical ophthalmic use
Contains no preservatives. 1 Vial 800 mg each HARROW ®
PRINCIPAL DISPLAY PANEL - Pouch
NDC 82667-300-01
Iheezo TM
(chloroprocaine hydrochloride ophthalmic gel) 3%
To be administered by physician only.
Single-Patient-Use Vial
Discard unused portion.
For topical ophthalmic use.
Contains no preservatives.
Sterile (outer surface of the vial not sterile)
LOT: 1234
EXP: YYYY-MM
Rx Only
Vial 800 mg