Uses
- ▪
- temporarily relieves these symptoms due to hay fever or other upper respiratory allergies:
- ▪
- runny nose
- ▪
- sneezing
- ▪
- itchy, watery eyes
- ▪
- itching of the nose or throat
Warnings
Do not use
- ▪
- to make a child sleepy
- ▪
- with any other product containing diphenhydramine, even one used on skin
Ask a doctor before use if the child has
- ▪
- a breathing problem such as chronic bronchitis
- ▪
- glaucoma
Directions
- •
- find right dose on chart below
- •
- take every 4 to 6 hours, or as directed by a doctor
- •
- do not take more than 6 doses in 24 hours
- •
- mL = milliliter
|
Age (yr) |
Dose (mL) |
|
children under 2 years |
do not use |
|
children 2 to 5 years |
do not use unless directed by a doctor |
|
children 6 to 11 years |
5 mL to 10 mL |
Attention: use only enclosed dosing cup designed for use with this product. Do not use any other dosing device.
Other information
- •
- each 5 mL contains: sodium 3 mg
- •
- store at room temperature)
- •
- protect from sunlight
- •
- see bottom panel for lot number and expiration date
- •
- contains low sodium
Inactive ingredients
anhydrous citric acid, carboxymethylcellulose sodium, flavors, glycerin, potassium citrate, purified water, sodium benzoate, sorbitol, sucralose.
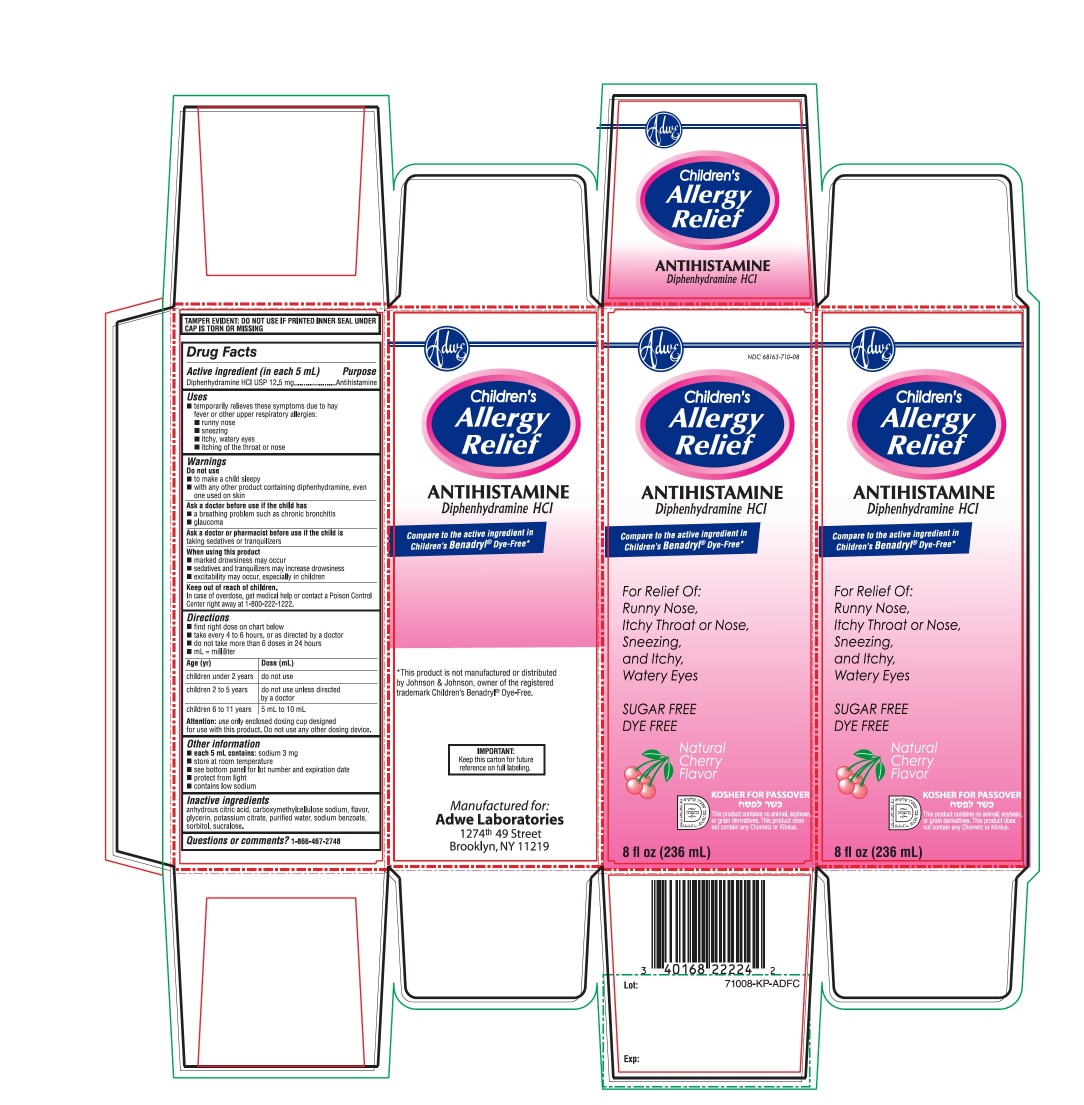
PRINCIPAL DISPLAY PANEL
NDC 68163-710-08
Compare to active ingredient in Children'sBenadryl® Dye-Free*
Children’s Allergy Relief
Antihistamine
Diphenhydramine HCl
For Relief Of:
- •
- Runny nose
- •
- sneezing
- •
- itchy, watery eyes
- •
- Itchy Throat or Nose
- Natural Cherry Flavor
8 fl oz (236 mL)
Manufactured for:
Adwe Laboratories
1274th 49 Street
Brooklyn, NY 11219
*This product is not manufactured or distributed by Johnson & Johnson, owner of the registered trademark Children’s Benadryl® Dye –Free.