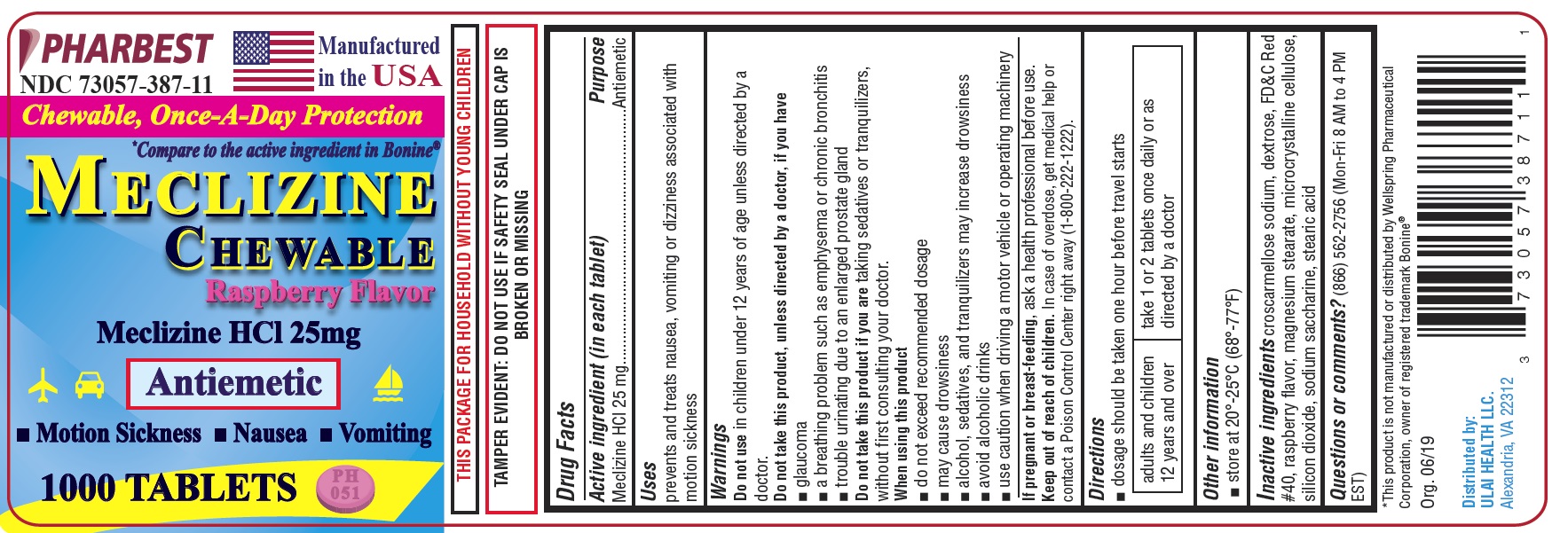
MECLIZINE- meclizine hcl 25mg tablet, chewable
Ulai Health LLC
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
Active ingredient (in each tablet)
Meclizine HCl 25mg
Uses
prevents and treats nausea, vomiting or dizziness associated with motion sickness
Do not use
in children under 12 years of age unless directed by a doctor.
Do not take this product, unless directed by a doctor, if you have
-
glaucoma
- a breathing problem such as emphysema or chronic bronchitis
- trouble urinating due to an enlarged prostate gland
Do not take this product if you are taking sedatives or tranquilizers, without first consulting your doctor.
When using this product
- do not exceed recommended dosage
- may cause drowsiness
- alcohol, sedatives, and tranquilizers may increase drowsiness
- avoid alcoholic drinks
- use causion when driving a motor vehicle or operating machinery
If pregnant or breast-feeding,
ask a health professional before use.
Keep out of reach of children.
In case of overdose, get medical help or contact a Poison Control Center right away (1-800-222-1222).
Directions
- dosage should be taken one hour before travel starts
| adults and children 12 years and over | take 1 or 2 tablets once daily or as directed by a doctor |
Other information
- store at 20o-25°C (68o-77°F)
Inactive ingredients
croscarmellose sodium, dextrose, FD&C Red#40, raspberry flavor, magnesium stearate, microcrystalline cellulose, silicon dioxide, sodium saccharine, stearic acid
Questions or comments?
(866) 562-2756 (Mon-Fri 8 AM to 4 PM EST)
PHARBEST
NDC 73057-387-11
Manufactured in the USA
Chewable, Once-A-Day Protection
*Compare to the active ingredient in Bonine®
MECLIZINE
CHEWABLE
Raspberry Flavor
Meclizine HCl 25mg
Antiemetic
• Motion Sickness • Nausea • Vomiting
1000 TABLETS