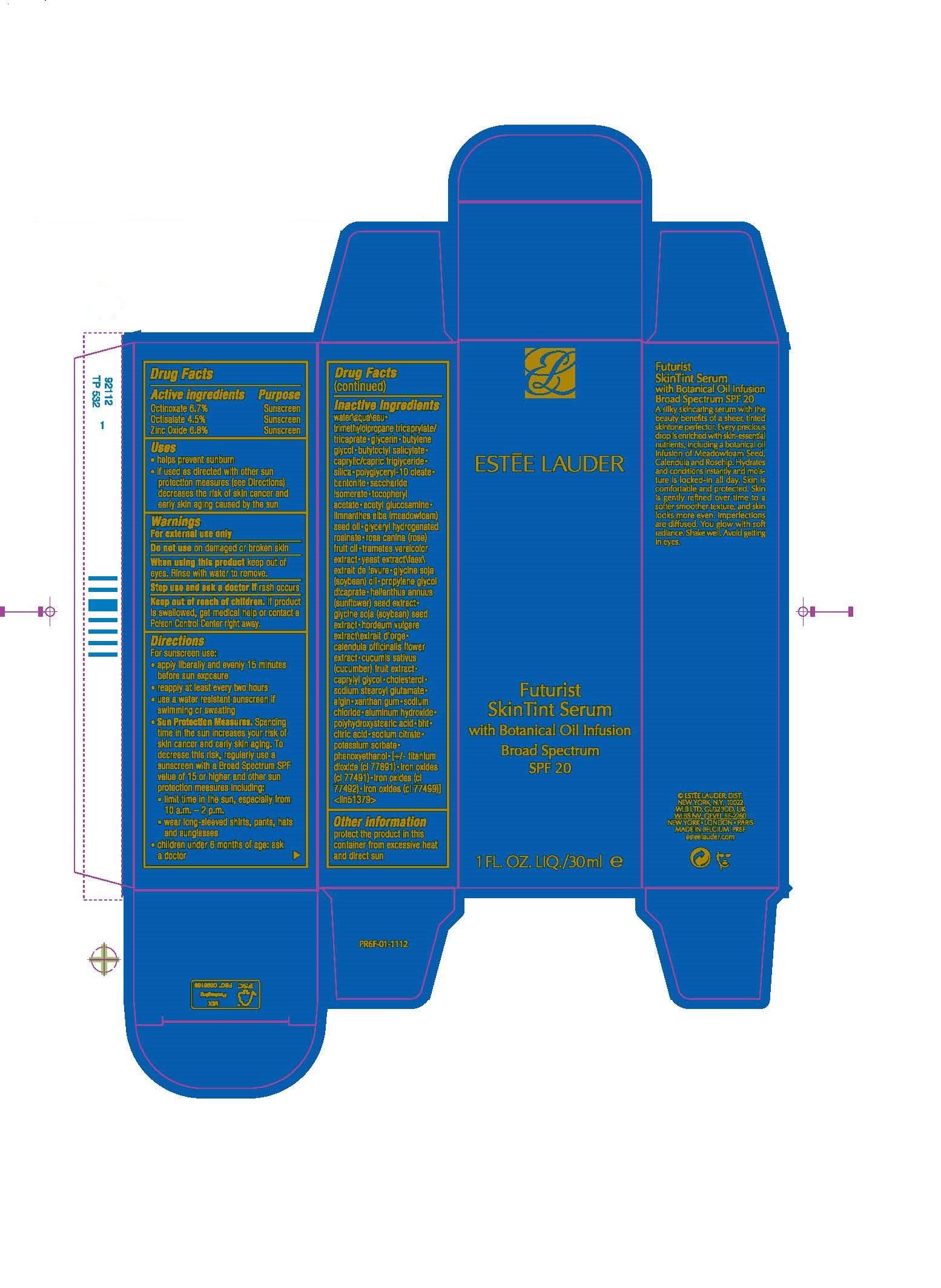
Directions
Directions For sunscreen use: • apply liberally and evenly 15 minutes before sun exposure • reapply at least every two hours • use a water resistant sunscreen if swimming or sweating • Sun Protection Measures. Spending time in the sun increases your risk of skin cancer and early skin aging. To decrease this risk, regularly use a sunscreen with a Broad Spectrum SPF value of 15 or higher and other sun protection measures including: • limit time in the sun, especially from 10 a.m. – 2 p.m. • wear long-sleeved shirts, pants, hats and sunglasses • children under 6 months of age: ask a doctor
Inactive Ingredients
WATER\AQUA\EAU [] TRIMETHYLOLPROPANE TRICAPRYLATE/TRICAPRATE [] GLYCERIN [] BUTYLENE GLYCOL [] BUTYLOCTYL SALICYLATE [] CAPRYLIC/CAPRIC TRIGLYCERIDE [] SILICA [] POLYGLYCERYL-10 OLEATE [] BENTONITE [] SACCHARIDE ISOMERATE [] TOCOPHERYL ACETATE [] ACETYL GLUCOSAMINE [] LIMNANTHES ALBA (MEADOWFOAM) SEED OIL [] GLYCERYL HYDROGENATED ROSINATE [] ROSA CANINA (ROSE) FRUIT OIL [] TRAMETES VERSICOLOR EXTRACT [] YEAST EXTRACT\FAEX\EXTRAIT DE LEVURE [] GLYCINE SOJA (SOYBEAN) OIL [] PROPYLENE GLYCOL DICAPRATE [] HELIANTHUS ANNUUS (SUNFLOWER) SEED EXTRACT [] GLYCINE SOJA (SOYBEAN) SEED EXTRACT [] HORDEUM VULGARE EXTRACT\EXTRAIT D'ORGE [] CALENDULA OFFICINALIS FLOWER EXTRACT [] CUCUMIS SATIVUS (CUCUMBER) FRUIT EXTRACT [] CAPRYLYL GLYCOL [] CHOLESTEROL [] SODIUM STEAROYL GLUTAMATE [] ALGIN [] XANTHAN GUM [] SODIUM CHLORIDE [] ALUMINUM HYDROXIDE [] POLYHYDROXYSTEARIC ACID [] BHT [] CITRIC ACID [] SODIUM CITRATE [] POTASSIUM SORBATE [] PHENOXYETHANOL [] [+/- TITANIUM DIOXIDE (CI 77891) [] IRON OXIDES (CI 77491) [] IRON OXIDES (CI 77492) [] IRON OXIDES (CI 77499)] <ILN51379>
 ESTEE LAUDER
ESTEE LAUDER