PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
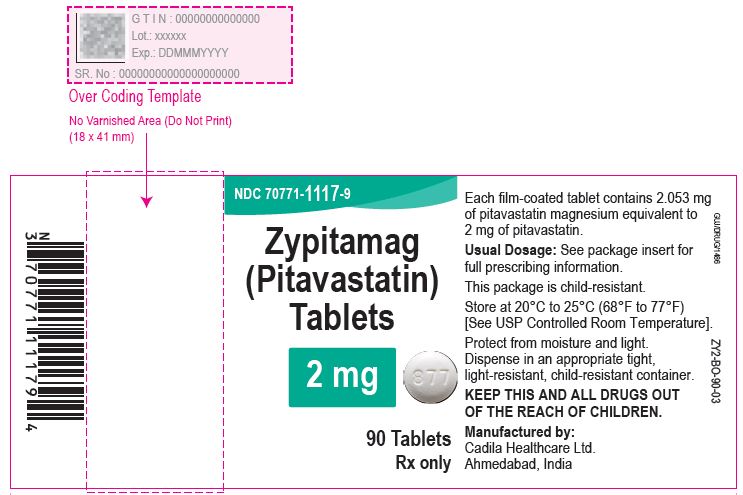
Zypitamag (Pitavastatin) Tablets, 2 mg
90 Tablets
Rx only
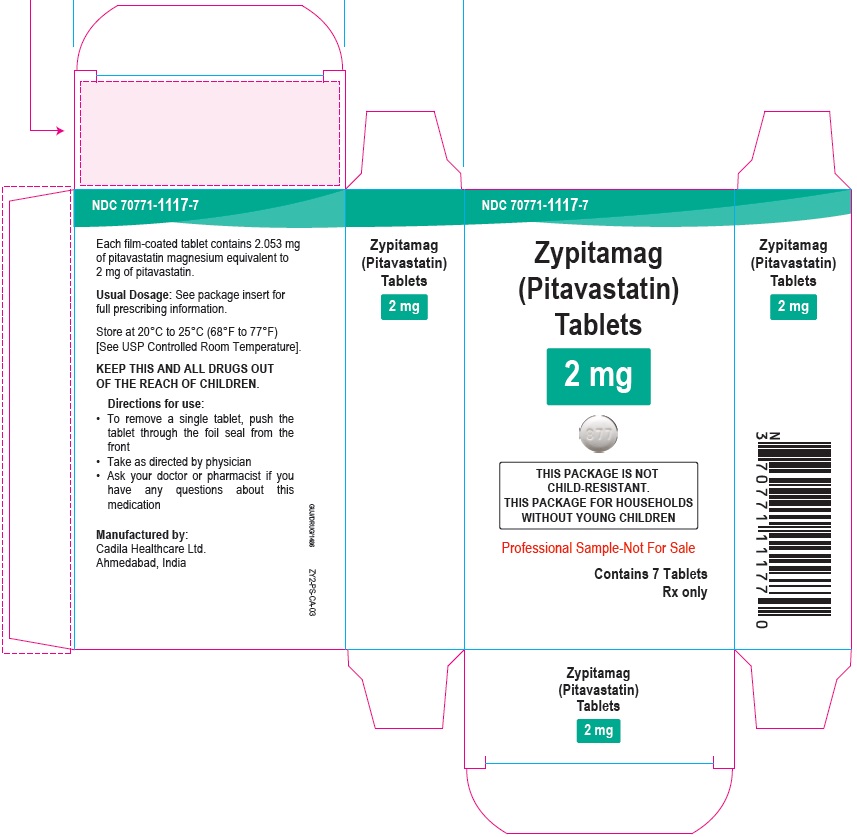
Zypitamag (Pitavastatin) Tablets, 2 mg
7 Tablets Blister Carton
Rx only
Professional Sample-Not For Sale
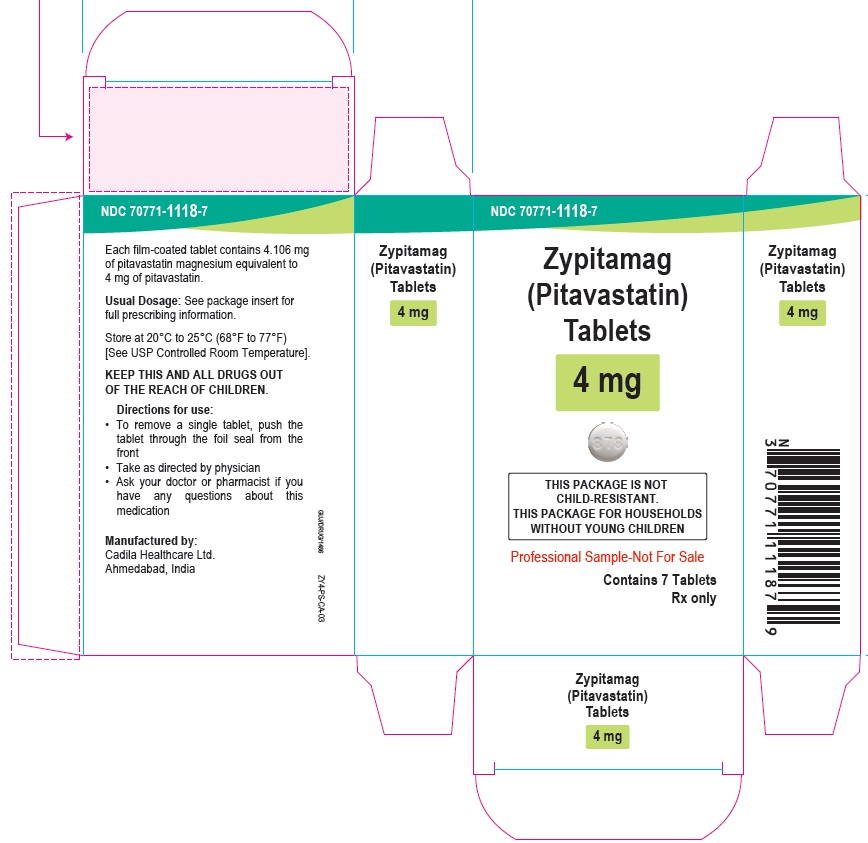
Zypitamag (Pitavastatin) Tablets, 4 mg
90 Tablets
Rx only
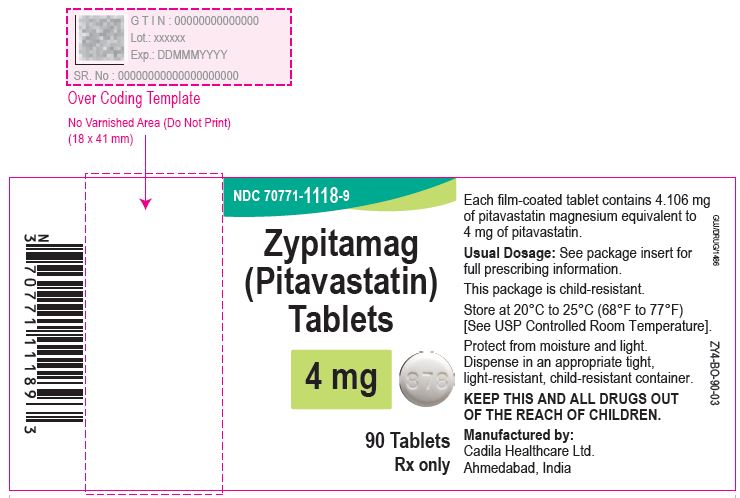
Zypitamag (Pitavastatin) Tablets, 4 mg
7 Tablets Blister Carton
Rx only
Professional Sample-Not For Sale