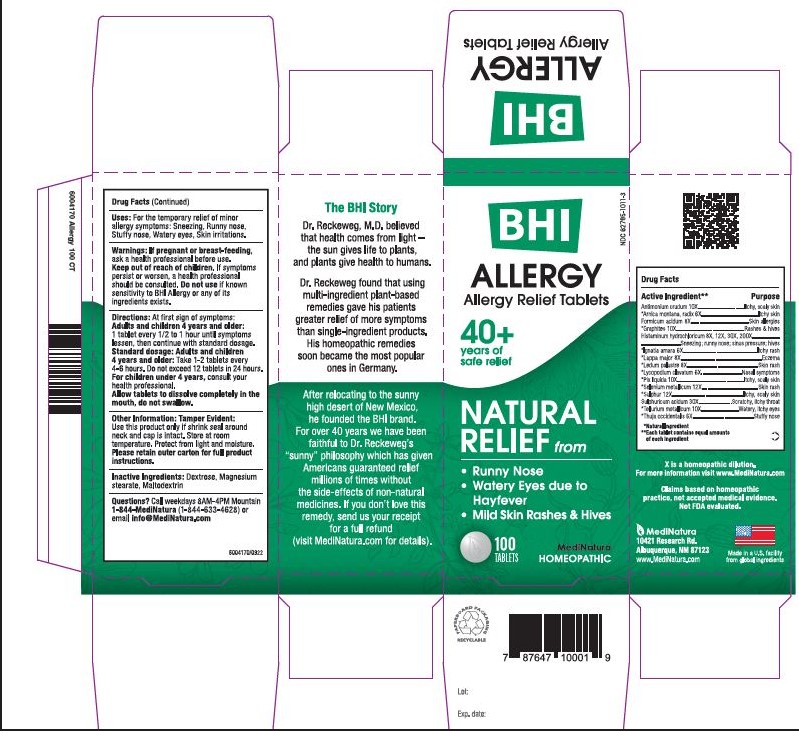
INDICATION AND USAGE
For the temporary relief of minor allergy symptoms: runny nose, watery eyes, skin irritations
WARNINGS
If pregnant or breast-feeding, ask a health professional before use. If symptoms persist or worsen, a healthcare professional should be consulted. Do not use if known sensitivity to BHI Allergy or any of its ingredients exists.
Directions
At first sign of symptoms: Adults and children 4 years and older:
1 tablet every 1/2 to 1 hour until symptoms lessen, then continue with standard dosage.
Standard dosage: Adults and children 4 years and older:
Take 1-2 tablets every 4 to 6 hours. Do not exceed 12 tablets in 24 hours.
For children under 4, consult your health professional.
Allow tablets to dissolve completely in the mouth, do not swallow.
ACTIVE INGREDIENTS
Active Ingredients:
Each tablet contains: Antimonium crudum 10X, *Arnica montana, radix 6X, Formicumacidum 8X, Graphites 10X, Histaminum hydrochloricum 8X, 12X, 30X, 200X, *Ignatia amara 6X, *Lappa major 8X, *Ledum palustre 8X, *Lycopodium clavatum 6X, *Pix liquida 10X, Selenium metallicum 12X, *Sulphur 12X, Sulphuricum acidum 30X, *Tellurium metallicum 10X, *Thuja occidentalis 6X 16.7 mg each.
*Natural ingredients