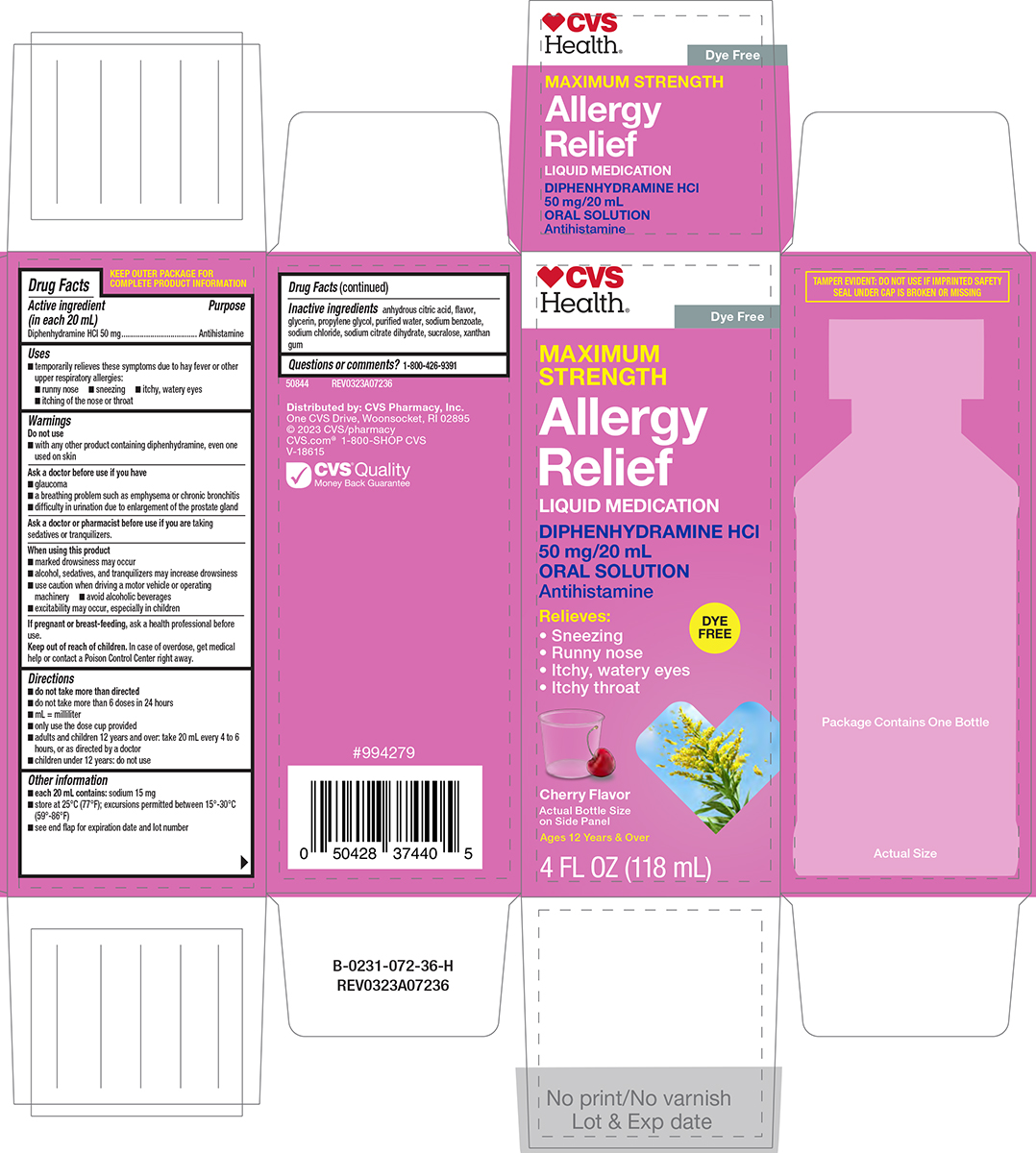
Uses
- temporarily relieves these symptoms due to hay fever or other upper respiratory allergies:
- runny nose
- sneezing
- itchy, watery eyes
- itching of the nose or throat
Warnings
Ask a doctor before use if you have
- glaucoma
- a breathing problem such as emphysema or chronic bronchitis
- difficulty in urination due to enlargement of the prostate gland
Directions
- do not take more than directed
- do not take more than 6 doses in 24 hours
- mL = milliliter
- only use the dose cup provided
- adults and children 12 years and over: take 20 mL every 4 to 6 hours, or as directed by a doctor
- children under 12 years: do not use
Other information
-
each 20 mL contains: sodium 15 mg
- store at 25°C (77°F); excursions permitted between 15°-30°C (59°-86°F)
- see end flap for expiration date and lot number
Inactive ingredients
anhydrous citric acid, flavor, glycerin, propylene glycol, purified water, sodium benzoate, sodium chloride, sodium citrate dihydrate, sucralose, xanthan gum
Principal display panel
♥CVS
Health®
Dye Free
MAXIMUM
STRENGTH
Allergy
Relief
LIQUID MEDICATION
DIPHENHYDRAMINE HCI
50 mg/20 mL
ORAL SOLUTION
Antihistamine
Relieves:
• Sneezing
• Runny nose
• Itchy, watery eyes
• Itchy throat
Cherry Flavor
Actual Bottle Size
on Side Panel
Ages 12 Years & Over
4 FL OZ (118 mL)
DYE
FREE
TAMPER EVIDENT: DO NOT USE IF IMPRINTED SAFETY
SEAL UNDER CAP IS BROKEN OR MISSING
50844 REV0323A07236
Distributed by: CVS Pharmacy, Inc.
One CVS Drive, Woonsocket, RI 02895
© 2023 CVS/pharmacy
CVS.com® 1-800-SHOP CVS
V-18615
CVS® Quality
Money Back Guarantee
CVS 44-072