|
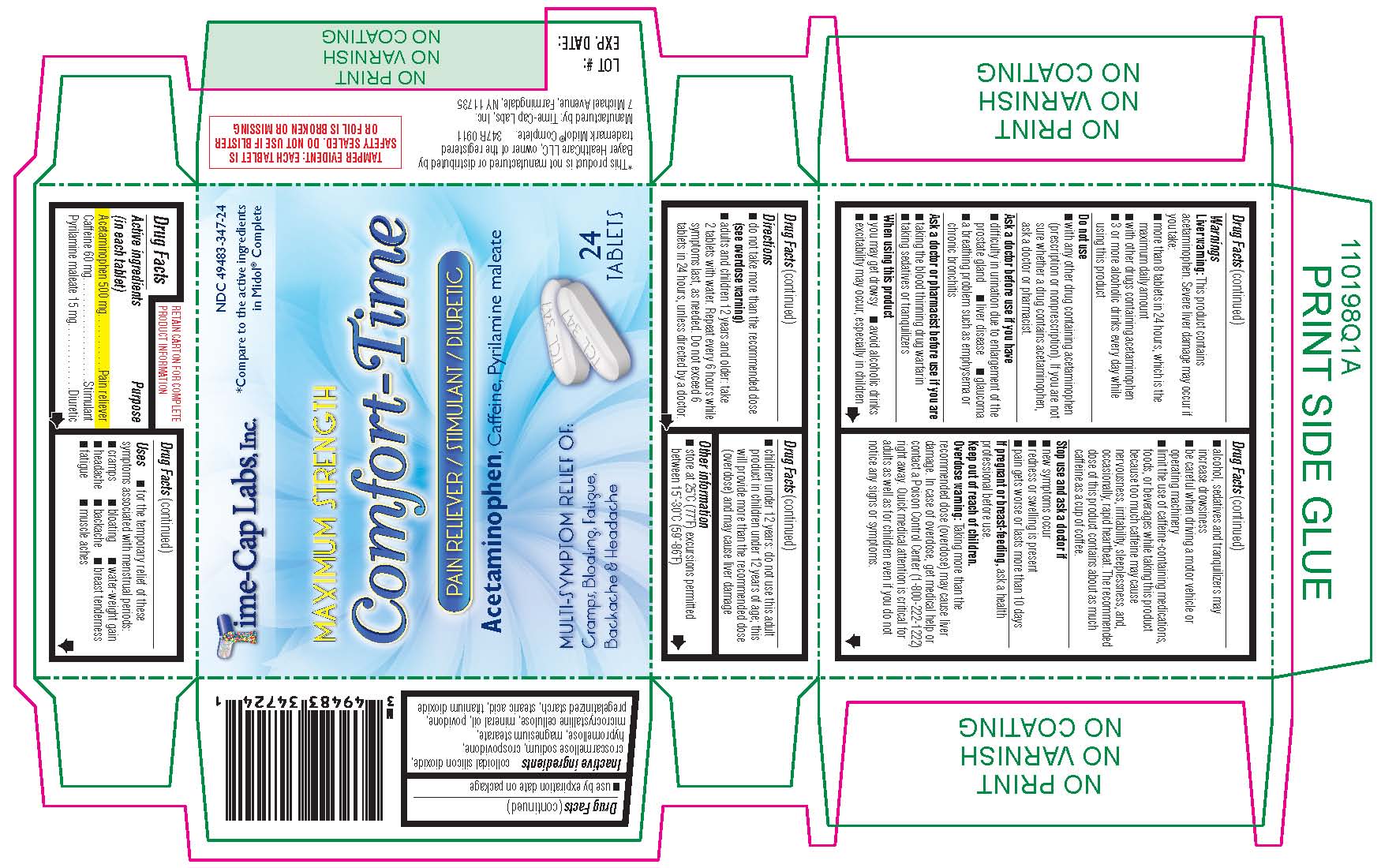
Uses for the temporary relief of these symptoms associated with menstrual periods: cramps
|
-
Warnings
Liver warning:This product contains acetaminophen. Severe liver damage may occur if you take:
more than 8 tablets in 24 hours, which is the maximum daily amount
with other drugs containing acetaminophen
3 or more alcoholic drinks every day while using this product
|
Do not use with any other drug containing acetaminophen (prescription or nonprescription). If you are not sure whether a drug contains acetaminophen, ask a doctor or pharmacist. |
|
Ask a doctor before use if you have difficulty in urination due to enlargement of the prostate gland
|
|
Ask a doctor or pharmacist before use if you are taking the blood thinning drug warfarin
|
|
When using this product you may get drowsy
|
|
Stop use and ask a doctor if new symptoms occur
|
|
WARNINGS
This product contains acetaminophen. Severe liver damage may occur if you take: more than 8 tablets in 24 hours, which is the maximum daily amount
|
|
Uses for the temporary relief of these symptoms associated with menstrual periods: cramps
|
|
Other information store at 25°C (77°F) excursions permitted between 15°-30°C (59°-86°F)
|