SOMINEX MAX- diphenhydramine hcl tablet
Medtech Products Inc.
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
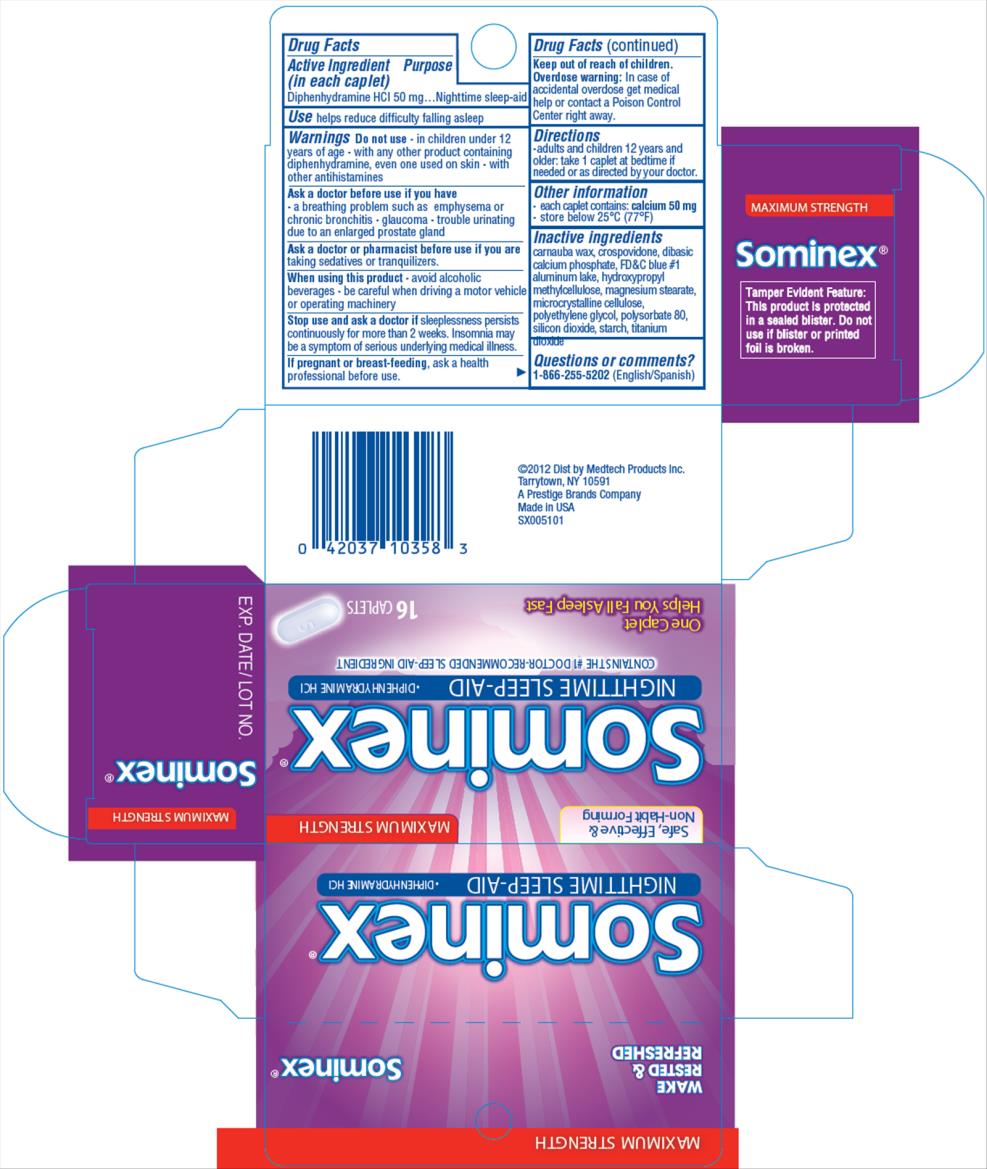
Active ingredient
(in each caplet)
Diphenhydramine HCl 50 mg
Purpose
Nighttime sleep-aid
Use
helps reduce difficultly falling asleep
Warnings
Do not use
- in children under 12 years of age
- with any other product containing diphenhydramine, even one used on skin
- with other antihistamines
Ask a doctor before use if you have
- a breathing problem such as emphysema or chronic bronchitis
- glaucoma
- trouble urinating due to an enlarged prostate gland
Ask a doctor or pharmacist before use if you are
taking sedatives or tranquilizers
When using this product
- avoid alcoholic beverages
- be careful when driving a motor vehicle or operating machinery
- drowsiness will occur
Stop use and ask a doctor if
sleeplessness persists continuously for more than 2 weeks. Insomnia may be a symptom of serious underlying medical illness.
If pregnant or breast-feeding,
ask a health professional before use.
Keep out of reach of children.
In case of overdose, get medical help or contact a Poison Control Center (1-800-222-1222) right away.
Directions
-
adults and children 12 years and older: take 1 caplet at bedtime if needed, or as directed by your doctor
Other information
store at room temperature 20º- 25ºC (68º-77ºF)
Inactive ingredients
croscarmellose sodium, dicalcium phosphate, FD&C blue no. 1 lake, hypromellose, lactose, magnesium stearate, microcrystalline cellulose, mineral oil, silica, stearate acid, talc, titanium dioxide, triacetin
Questions?
1-866-255-5202
PRINCIPAL DISPLAY PANEL
MAXIMUM STRENGTH FORMULA
Sominex®
NIGHTTIME SLEEP-AID DIPHENHYDRAMINE HCl
16 TABLETS
Medtech Products Inc.
