ACTIVE INGREDIENTS
Active ingredients (HPUS)
Carbo vegetabilis 12X
Chamomilla 12X
Foeniculum vulgare 12X
Lycopodium 30X
Nux moschata 12X
Raphanus sativus 12X
Zingiber officinale 12X
Cell Salts
Kali muriaticum 6X
Magnesia phosphorica 12X
Natrum phosphoricum 6X
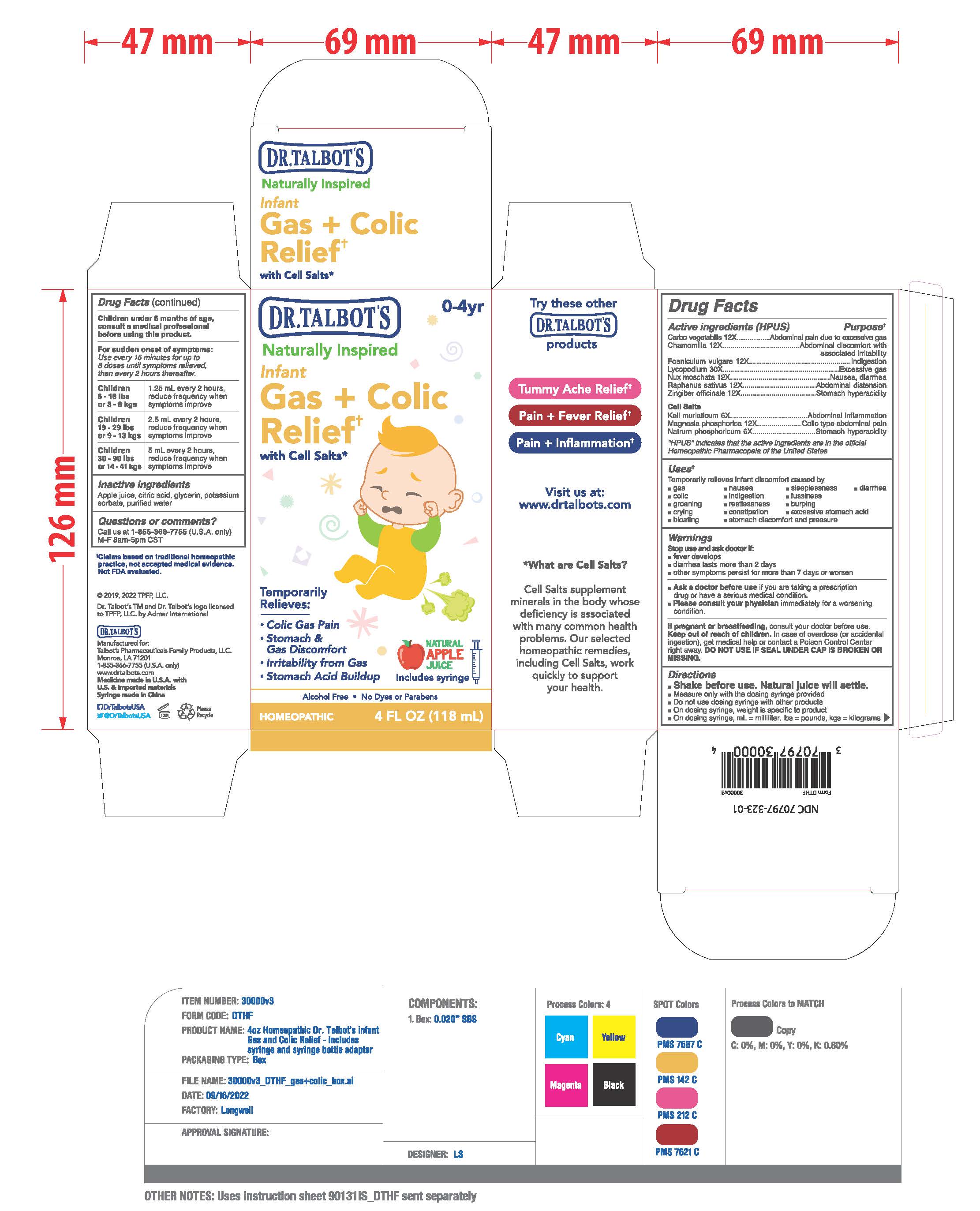
INACTIVE INGREDIENTS
Inactive ingredients
Apple juice, citric acid, glycerin, potassium
sorbate, purified water

ASK A DOCTOR
Ask a doctor before use if you are taking a prescription drugs or have a serious medical condition.
Please consult your physician immiediately for a worsening condition.

KEEP OUT OF REACH OF CHILDREN
Keep out of reach of children. In case of overdose (or accidental ingestion), get medical help or contact a Poison Control Center right away.
DO NOT USE IF SEAL UNDER CAP IS BROKEN OR MISSING.

DOSAGE
Children under 6 months of age, consult a medical professional before using this product.
For sudden onset of symptoms: Use every 15 minutes for up to 8 doses until symptoms relieved, every 2 hours thereafter.

Uses
Uses: Temporarily relieves infant discomfort caused by
- gas
- nausea
- fussiness
- colic
- indigestion
- burping
- groaning
- restlesness
- sleeplessness
- diarrhea
- crying
- constipation
- ecxessive stomach acid
- bloating
- stomach discomfort and presure

PURPOSE
Temprorily Relieves:
- Colic Gas Pain
- Stomach & Gas Discomfort
- Irritability from Gas
-
Stomach Acid Buildup
