ACTIVE INGREDIENTS
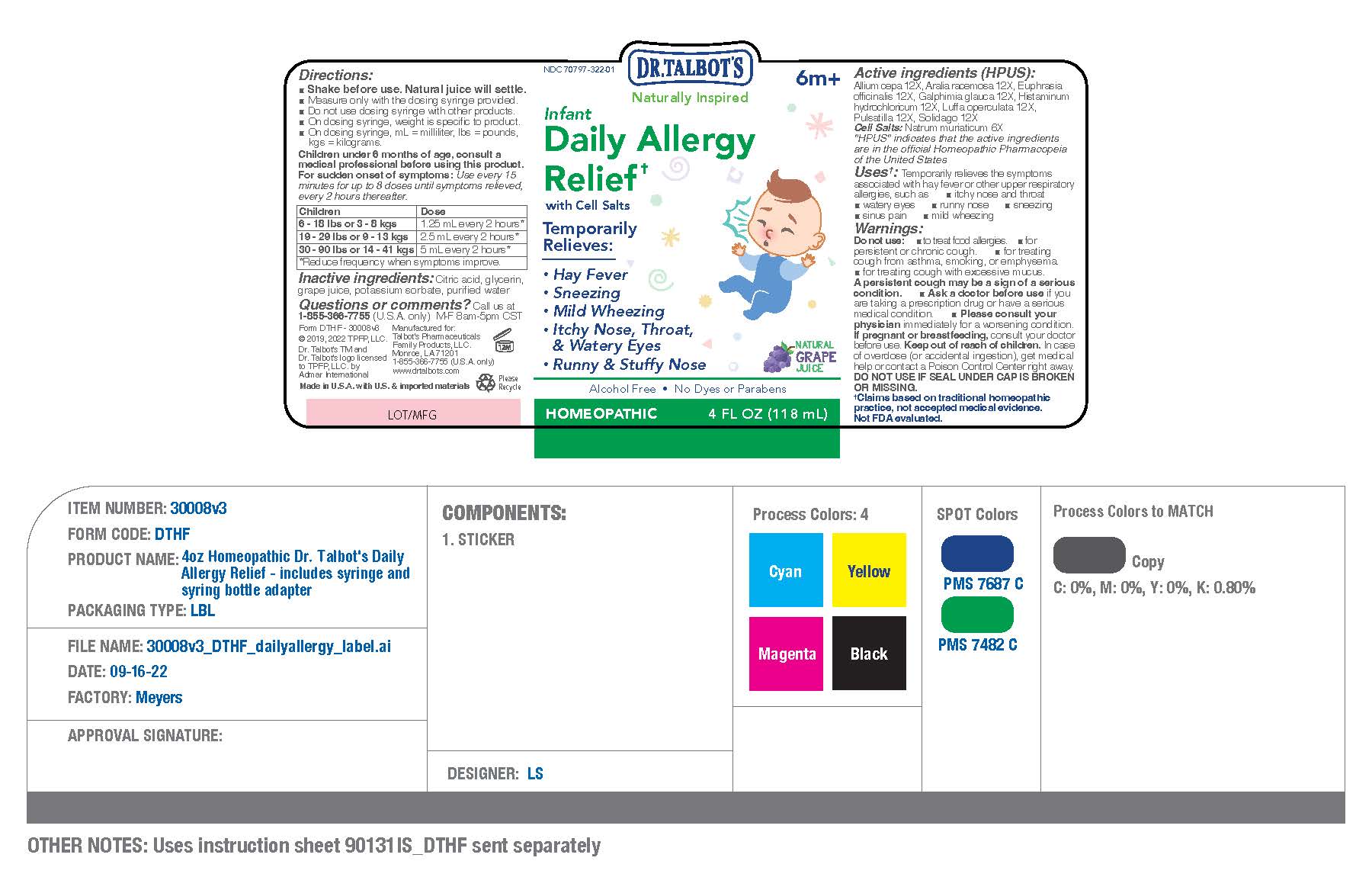 Active ingredients (HPUS)
Active ingredients (HPUS)
Allium cepa 12X
Aralia racemosa 12X
Euphrasia officinalis 12X.
Galphimia glauca 12X
Histaminum hydrochloricum 12X
Luffa operculata 12X
Pulsatilla 12X
Solidago 12X
CELL SALTS
Natrum muriaticum 6X
INACTIVE INGREDIENTS
 Inactive ingredients
Inactive ingredients
Citric acid, glycerin, grape juice, potassium
sorbate, purified water
PURPOSE
Temporarily Relieves:
- Hay Fever
- Sneezing
- Mild wheezing
- Itchy Nose, Thorat, & Watery Eyes
- Runny & Stuffy Nose

PREGNANCY
 If pregnant or breastfeeding, consult your doctor before use.
If pregnant or breastfeeding, consult your doctor before use.
Keep out of reach of children.
DIRECTIONS
 For sudden onset of symptoms:
For sudden onset of symptoms:
Use every 15 minutes for up to
8 doses until symptoms relieved,
then every 2 hours thereafter.
Children
6 - 18 lbs
or 3 - 8 kgs
1.25 mL every 2 hours,
reduce frequency when
symptoms improve
Children
19 - 29 lbs
or 9 - 13 kg
2.5 mL every 2 hours,
reduce frequency when
symptoms improve
Children
30 - 90 lbs
or 14 - 41 kgs
5 mL every 2 hours,
reduce frequency when
symptoms improve
ASK A DOCTOR
Ask a doctor before use if you are taking a prescription drug or have a serious medical condition.
Please consult your physician immidiately for a worsening condition.

Warnings
Warnings:
Do not use:
- to treat food allergies
- for persistent or chronic cough
- for treating cough from asthma, smoking, or emphysema
- for treating cough with excessive mucus
A persistent cough may be a sign of a serious condition.
Uses
Temporrarily relieves the symptoms asscociated with hay fever or other upper respiratory allergies, such as
- itchy nose and throat
- watery eyes
- runny nose
- sneezing
- sinus pain
- mild wheezing
