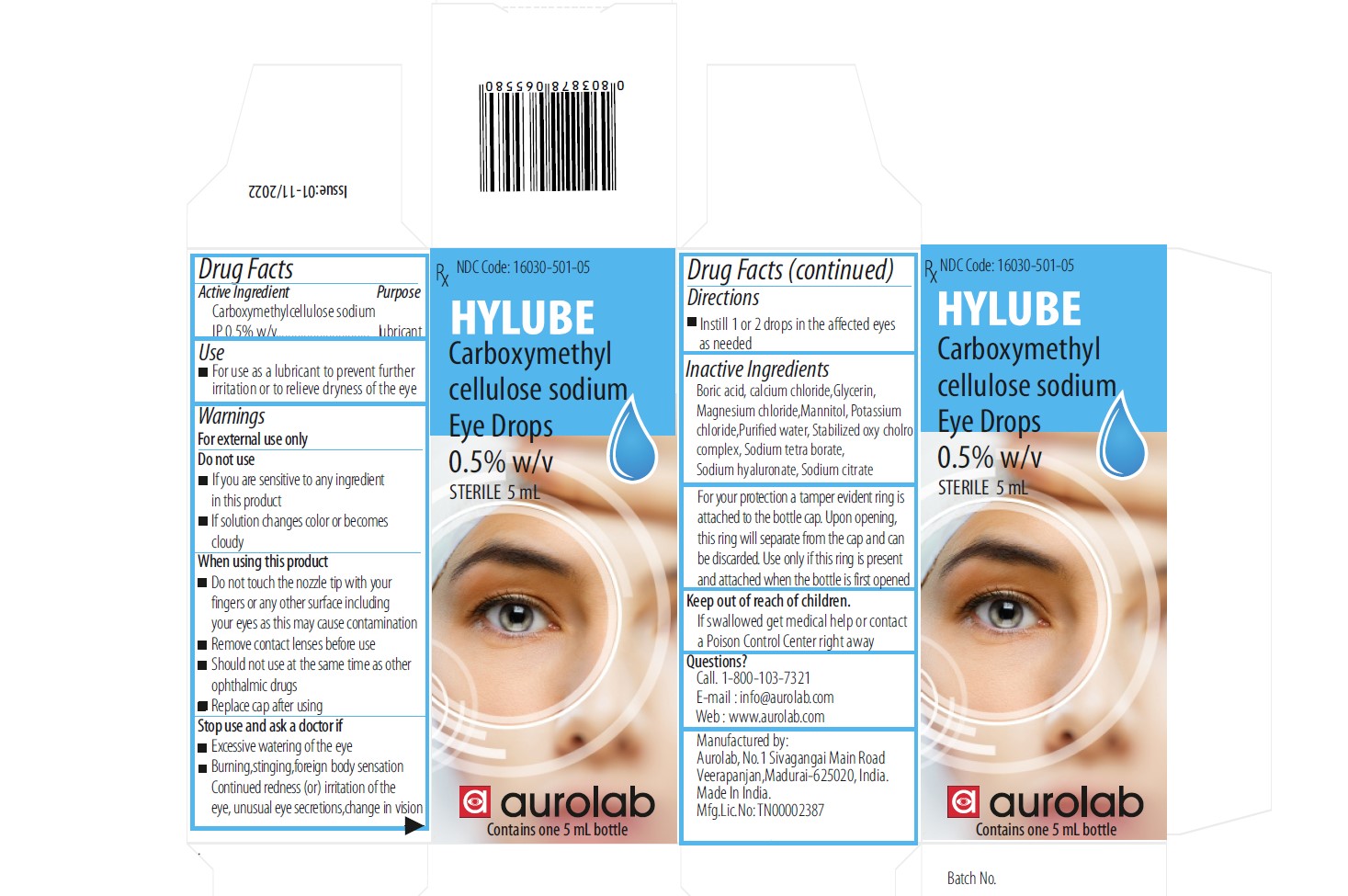
CARBOXYMETHYLCELLULOSE SODIUM EYE DROPS 0.5%- carboxymethylcellulose sodium eye drops 0.5% for solution
Aurolab
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
Active ingredient
Carboxymethyl cellulose sodium Eye Drops 0.5% w/v
DIRECTIONS FOR USE
- lnstill 1or 2 drops in the affected eye, as needed
INACTIVE INGREDIENT
1.Boric acid
2.Calcium chloride
3.Glycerin
4.Magnesium chloride
5.Mannitol
6.Potassium chloride
7.Purified water
8.Stablized oxy cholro complex
9.Sodium tetra borate
10.Sodium hyaluronate
11.Sodium citrate
Tamper Protection
- For your protection a tamper evident ring is attached to the bottlecap
- Upon opening, this will separate from the cap and can be discarded
- Use only if this ring is present and attached when the bottle is first opened
Use
For use as a lubricant to prevent further irritation or to relieve dryness of the eye
Questions
Call. 1-800-103-7321
E-mail : info@aurolab.com
Web : www.aurolab.com
Keep out of reach of children
If swallowed get medical help or contact a Poison Control Center right away
Stop use and ask a doctor if
1.Excessive watering of the eye
2.Burning, stinging,foreign body sensation Continued redness (or) irritation of the eye, unusual eye secretions, change in vision
Do not use
1.If you are sensitive to any ingredient in this product
2.If solution changes color or becomes cloudy
Warnings
For external use only
Indication & usage
Do not touch the nozzle tip to any surface since this may contaminate the solution
Remove contact lenses before use Should not use at the same time as other ophthalmic drugs
Replace cap after using
Dose
Instill 1 or 2 drops in the affected eyes as needed
Eye lubricant
Eye lubricant
Carton