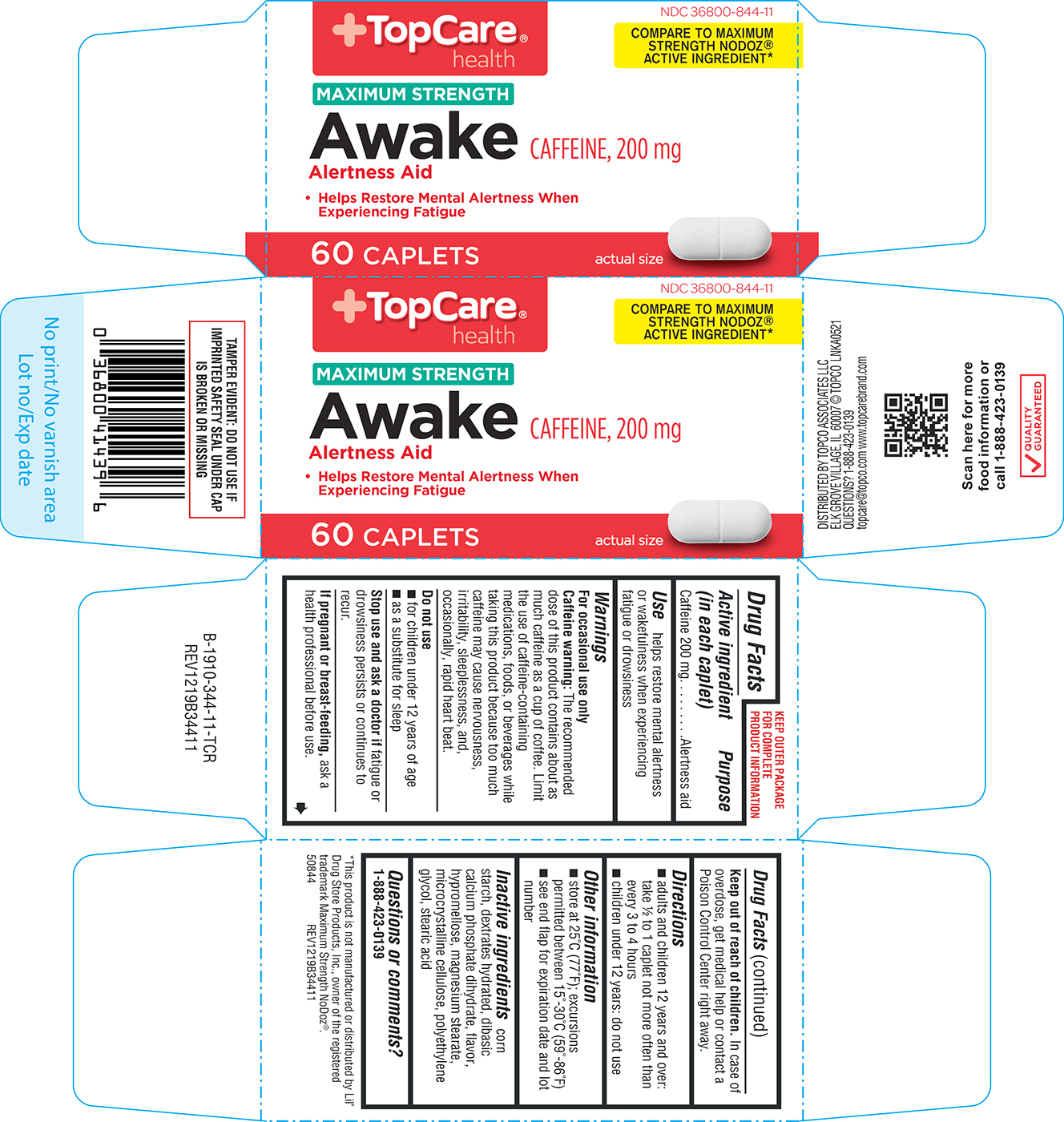
Warnings
For occasional use only
Caffeine warning: The recommended dose of this product contains about as much caffeine as a cup of coffee. Limit the use of caffeine-containing medications, foods, or beverages while taking this product because too much caffeine may cause nervousness, irritability, sleeplessness, and, occasionally, rapid heart beat.
Directions
- adults and children 12 years and over: take ½ to 1 caplet not more often than every 3 to 4 hours
- children under 12 years: do not use
Other information
- store at 25°C (77°F); excursions permitted between 15°-30°C (59°-86°F)
- see end flap for expiration date and lot number
Inactive ingredients
corn starch, dextrates hydrated, dibasic calcium phosphate dihydrate, flavor, hypromellose, magnesium stearate, microcrystalline cellulose, polyethylene glycol, stearic acid
Principal display panel
TopCare®
health
NDC 36800-844-11
COMPARE TO MAXIMUM
STRENGTH NODOZ®
ACTIVE INGREDIENT*
MAXIMUM STRENGTH
Awake CAFFEINE, 200 mg
Alertness Aid
• Helps Restore Mental Alertness When
Experiencing Fatigue
60 CAPLETS
actual
size
TAMPER EVIDENT: DO NOT USE IF
IMPRINTED SAFETY SEAL UNDER CAP
IS BROKEN OR MISSING
*This product is not manufactured or distributed by Lil’
Drug Store Products, Inc., owner of the registered
trademark Maximum Strength NoDoz®.
50844 REV1219B3441
DISTRIBUTED BY TOPCO ASSOCIATES LLC
ELK GROVE VILLAGE, IL 60007 © TOPCO LNKA0521
QUESTIONS? 1-888-423-0139
topcare@topco.com www.topcarebrand.com
QUALITY
GUARANTEED
TopCare 44-344