DESCRIPTION
-
Metaxalone is available as an 800 mg, light pink to pink, capsule shaped, scored uncoated tablet.
Chemically, metaxalone is 5-[(3,5- dimethylphenoxy) methyl]-2-oxazolidinone. The empirical formula is C12H15NO3, which corresponds to a molecular weight of 221.25. The structural formula is:
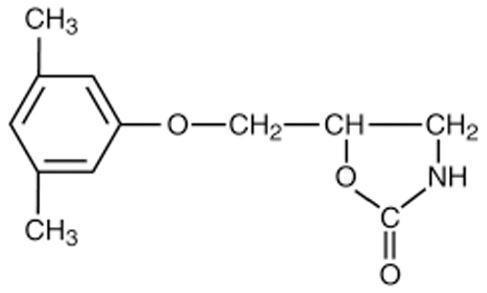
Metaxalone is a white to almost white, odorless crystalline powder freely soluble in chloroform, soluble in methanol and in 96% ethanol, but practically insoluble in ether or water.
Each tablet contains 800 mg metaxalone and the following inactive ingredients: calcium carbonate, FD&C Red #40, hypromellose, lactose monohydrate, microcrystalline cellulose, povidone, silicone dioxide, sodium starch glycolate and sodium stearyl fumarate.
CLINICAL PHARMACOLOGY
- Mechanism of Action
The mechanism of action of metaxalone in humans has not been established, but may be due to general central nervous system depression. Metaxalone has no direct action on the contractile mechanism of striated muscle, the motor end plate or the nerve fiber.
PharmacokineticsThe pharmacokinetics of metaxalone have been evaluated in healthy adult volunteers after single dose administration of metaxalone under fasted and fed conditions at doses ranging from 400 mg to 800 mg.
AbsorptionPeak plasma concentrations of metaxalone occur approximately 3 hours after a 400 mg oral dose under fasted conditions. Thereafter, metaxalone concentrations decline log-linearly with a terminal half-life of 9.0 ± 4.8 hours. Doubling the dose of metaxalone from 400 mg to 800 mg results in a roughly proportional increase in metaxalone exposure as indicated by peak plasma concentrations (Cmax) and area under the curve (AUC). Dose proportionality at doses above 800 mg has not been studied. The absolute bioavailability of metaxalone is not known.
The single-dose pharmacokinetic parameters of metaxalone in two groups of healthy volunteers are shown in Table 1.
Table 1: Mean (%CV) Metaxalone Pharmacokinetic Parameters
Food EffectsDose (mg)
Cmax
(ng/mL)
Tmax (h)
AUC∞
(ng•h/mL)
t½(h)
CL/F
(L/h)
4001
983 (53)
3.3 (35)
7479 (51)
9.0 (53)
68 (50)
8002
1816 (43)
3.0 (39)
15044 (46)
8.0 (58)
66 (51)
1Subjects received 1x400 mg tablet under fasted conditions (N=42)
2Subjects received 2x400 mg tablets under fasted conditions (N=59)
A randomized, two-way, crossover study was conducted in 42 healthy volunteers (31 males, 11 females) administered one 400 mg metaxalone tablet under fasted conditions and following a standard high-fat breakfast. Subjects ranged in age from 18 to 48 years (mean age = 23.5 ± 5.7 years). Compared to fasted conditions, the presence of a high fat meal at the time of drug administration increased Cmax by 177.5% and increased AUC (AUC0-t, AUC∞) by 123.5% and 115.4%, respectively. Time-to-peak concentration (Tmax) was also delayed (4.3 h versus 3.3 h) and terminal half-life was decreased (2.4 h versus 9.0 h) under fed conditions compared to fasted.
In a second food effect study of similar design, two 400 mg metaxalone tablets (800 mg) were administered to healthy volunteers (N=59, 37 males, 22 females), ranging in age from 18 to 50 years (mean age = 25.6± 8.7 years). Compared to fasted conditions, the presence of a high fat meal at the time of drug administration increased Cmax by 193.6% and increased AUC (AUC0-t, AUC∞) by 146.4% and 142.2%, respectively. Time-to-peak concentration (Tmax) was also delayed (4.9 h versus 3.0 h) and terminal half-life was decreased (4.2 h versus 8.0 h) under fed conditions compared to fasted conditions. Similar food effect results were observed in the above study when one metaxalone 800 mg tablet was administered in place of two metaxalone 400 mg tablets. The increase in metaxalone exposure coinciding with a reduction in half-life may be attributed to more complete absorption of metaxalone in the presence of a high fat meal (Figure 1).
Distribution, Metabolism, and Excretion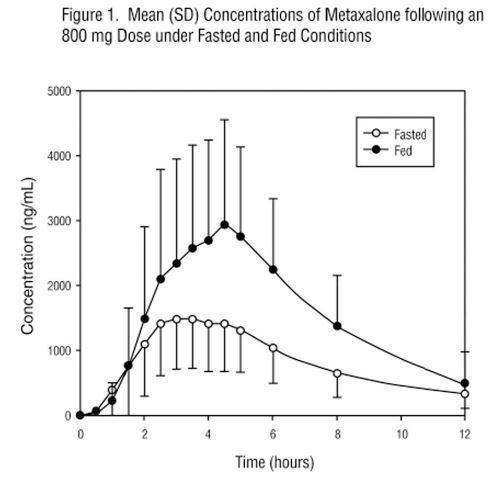
Although plasma protein binding and absolute bioavailability of metaxalone are not known, the apparent volume of distribution (V/F ~ 800 L) and lipophilicity (log P = 2.42) of metaxalone suggest that the drug is extensively distributed in the tissues. Metaxalone is metabolized by the liver and excreted in the urine as unidentified metabolites. Hepatic Cytochrome P450 enzymes play a role in the metabolism of metaxalone. Specifically, CYP1A2, CYP2D6, CYP2E1, and CYP3A4 and, to a lesser extent, CYP2C8, CYP2C9, and CYP2C19 appear to metabolize metaxalone.
Metaxalone does not significantly inhibit major CYP enzymes such as CYP1A2, CYP2A6, CYP2B6, CYP2C8, CYP2C9, CYP2C19, CYP2D6, CYP2E1, and CYP3A4. Metaxalone does not significantly induce major CYP enzymes such as CYP1A2, CYP2B6, and CYP3A4 in vitro.
Pharmacokinetics in Special PopulationsAge:
The effects of age on the pharmacokinetics of metaxalone were determined following single administration of two 400 mg tablets (800 mg) under fasted and fed conditions. The results were analyzed separately, as well as in combination with the results from three other studies. Using the combined data, the results indicate that the pharmacokinetics of metaxalone are significantly more affected by age under fasted conditions than under fed conditions, with bioavailability under fasted conditions increasing with age.
The bioavailability of metaxalone under fasted and fed conditions in three groups of healthy volunteers of varying age is shown in Table 2.
Table 2: Mean (%CV) Pharmacokinetic Parameters Following Single Administration of Two 400 mg Metaxalone Tablets (800 mg) under Fasted and Fed Conditions
Younger Volunteers
Older Volunteers
Age (years)
25.6 ± 8.7
39.3 ± 10.8
71.5 ± 5.0
N
59
21
23
Food
Fasted
Fed
Fasted
Fed
Fasted
Fed
Cmax (ng/mL)
1816
(43)
3510
(41)
2719
(46)
2915
(55)
3168
(43)
3680
(59)
Tmax (h)
3.0
(39)
4.9
(48)
3.0
(40)
8.7
(91)
2.6
(30)
6.5
(67)
AUC0-t (ng·h/mL)
14531
(47)
20683
(41)
19836
(40)
20482
(37)
23797
(45)
24340
(48)
AUC∞ (ng·h/mL)
15045
(46)
20833
(41)
20490
(39)
20815
(37)
24194
(44)
24704
(47)
Gender:
The effect of gender on the pharmacokinetics of metaxalone was assessed in an open label study, in which 48 healthy adult volunteers (24 males, 24 females) were administered two metaxalone 400 mg tablets (800 mg) under fasted conditions. The bioavailability of metaxalone was significantly higher in females compared to males as evidenced by Cmax (2115 ng/mL versus 1335 ng/mL) and AUC∞ (17884 ng·h/mL versus 10328 ng·h/mL). The mean half-life was 11.1 hours in females and 7.6 hours in males. The apparent volume of distribution of metaxalone was approximately 22% higher in males than in females, but not significantly different when adjusted for body weight. Similar findings were also seen when the previously described combined dataset was used in the analysis.
Hepatic/Renal Insufficiency:
The impact of hepatic and renal disease on the pharmacokinetics of metaxalone has not been determined. In the absence of such information, metaxalone should be used with caution in patients with hepatic and/or renal impairment.
INDICATIONS AND USAGE
-
Metaxalone is indicated as an adjunct to rest, physical therapy, and other measures for the relief of discomforts associated with acute, painful musculoskeletal conditions. The mode of action of this drug has not been clearly identified, but may be related to its sedative properties. Metaxalone does not directly relax tense skeletal muscles in man.
CONTRAINDICATIONS
-
Known hypersensitivity to any components of this product.
Known tendency to drug induced, hemolytic, or other anemias.
Significantly impaired renal or hepatic function.
PRECAUTIONS
-
Metaxalone should be administered with great care to patients with pre-existing liver damage. Serial liver function studies should be performed in these patients.
False-positive Benedict’s tests, due to an unknown reducing substance, have been noted. A glucose-specific test will differentiate findings.
Taking metaxalone with food may enhance general CNS depression; elderly patients may be especially susceptible to this CNS effect. (See CLINICAL PHARMACOLOGY: Pharmacokinetics and PRECAUTIONS: Information for Patients section).
Information for PatientsMetaxalone may impair mental and/or physical abilities required for performance of hazardous tasks, such as operating machinery or driving a motor vehicle, especially when used with alcohol or other CNS depressants.
Drug InteractionsThe sedative effects of metaxalone and other CNS depressants (e.g., alcohol, alcohol, benzodiazepines, opioids, tricyclic antidepressants) may be additive. Therefore, caution should be exercised with patients who take more than one of these CNS depressants simultaneously.
Carcinogenesis, Mutagenesis, Impairment of FertilityThe carcinogenic potential of metaxalone has not been determined.
PregnancyReproduction studies in rats have not revealed evidence of impaired fertility or harm to the fetus due to metaxalone. Post marketing experience has not revealed evidence of fetal injury, but such experience cannot exclude the possibility of infrequent or subtle damage to the human fetus. Safe use of metaxalone has not been established with regard to possible adverse effects upon fetal development. Therefore, metaxalone tablets should not be used in women who are or may become pregnant and particularly during early pregnancy unless, in the judgement of the physician, the potential benefits outweigh the possible hazards.
Nursing MothersIt is not known whether this drug is secreted in human milk. As a general rule, nursing should not be undertaken while a patient is on a drug since many drugs are excreted in human milk.
Pediatric UseSafety and effectiveness in children 12 years of age and below have not been established.
ADVERSE REACTIONS
-
The most frequent reactions to metaxalone include:
CNS: drowsiness, dizziness, headache, and nervousness or “irritability”;
Digestive: nausea, vomiting, gastrointestinal upset.
Other adverse reactions are:
Immune System: hypersensitivity reaction, rash with or without pruritus;
Hematologic: leukopenia; hemolytic anemia;
Hepatobiliary: jaundice.
Though rare, anaphylactoid reactions have been reported with metaxalone.
OVERDOSAGE
-
Deaths by deliberate or accidental overdose have occurred with metaxalone, particularly in combination with antidepressants, and have been reported with this class of drug in combination with alcohol.
When determining the LD50 in rats and mice, progressive sedation, hypnosis and finally respiratory failure were noted as the dosage increased. In dogs, no LD50 could be determined as the higher doses produced an emetic action in 15 to 30 minutes.
Treatment - Gastric lavage and supportive therapy. Consultation with a regional poison control center is recommended.
