WARNING
Because midodrine hydrochloride tablets can cause marked elevation of supine blood pressure, it should be used in patients whose lives are considerably impaired despite standard clinical care. The indication for use of midodrine hydrochloride tablets in the treatment of symptomatic orthostatic hypotension is based primarily on a change in a surrogate marker of effectiveness, an increase in systolic blood pressure measured one minute after standing, a surrogate marker considered likely to correspond to a clinical benefit. At present, however, clinical benefits of midodrine hydrochloride tablets, principally improved ability to carry out activities of daily living, have not been verified.
DESCRIPTION
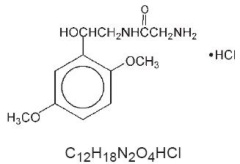
Midodrine hydrochloride is a vasopressor/antihypotensive. The chemical name for midodrine hydrochloride is acetamide, 2-amino- N-[2-(2,5-dimethoxyphenyl)-2-hydroxyethyl]-, monohydrochloride, (±)-. The molecular weight of midodrine hydrochloride is 290.7. Its structural formula and molecular formula are:
Midodrine hydrochloride, USP is an odorless, white, crystalline powder. It is soluble in water and sparingly soluble in methanol and has a pKa of 7.8 (0.3% aqueous solution) and a pH of 3.5 to 5.5 (5% aqueous solution). It has a melting range of 200° to 203°C.
Each midodrine hydrochloride tablet, USP for oral administration contains 5 mg of midodrine hydrochloride, USP. In addition, each tablet contains the following inactive ingredients: magnesium stearate, microcrystalline cellulose, and pregelatinized starch.
CLINICAL PHARMACOLOGY
Mechanism of Action
Midodrine hydrochloride tablets form an active metabolite, desglymidodrine, that is an alpha 1-agonist, and exerts its actions via activation of the alpha-adrenergic receptors of the arteriolar and venous vasculature, producing an increase in vascular tone and elevation of blood pressure. Desglymidodrine does not stimulate cardiac beta-adrenergic receptors. Desglymidodrine diffuses poorly across the blood-brain barrier, and is therefore not associated with effects on the central nervous system.
Administration of midodrine hydrochloride tablets results in a rise in standing, sitting, and supine systolic and diastolic blood pressure in patients with orthostatic hypotension of various etiologies. Standing systolic blood pressure is elevated by approximately 15 mmHg to 30 mmHg at 1 hour after a 10 mg dose of midodrine, with some effect persisting for 2 to 3 hours. Midodrine hydrochloride tablets have no clinically significant effect on standing or supine pulse rates in patients with autonomic failure.
Pharmacokinetics
Midodrine hydrochloride tablets are a prodrug, i.e., the therapeutic effect of orally administered midodrine is due to the major metabolite desglymidodrine, formed by deglycination of midodrine. After oral administration, midodrine hydrochloride tablets are rapidly absorbed. The plasma levels of the prodrug peak after about half an hour, and decline with a half-life of approximately 25 minutes, while the metabolite reaches peak blood concentrations about 1 to 2 hours after a dose of midodrine and has a half-life of about 3 to 4 hours. The absolute bioavailability of midodrine (measured as desglymidodrine) is 93%. The bioavailability of desglymidodrine is not affected by food. Approximately the same amount of desglymidodrine is formed after intravenous and oral administration of midodrine. Neither midodrine nor desglymidodrine is bound to plasma proteins to any significant extent.
Metabolism and Excretion
Thorough metabolic studies have not been conducted, but it appears that deglycination of midodrine to desglymidodrine takes place in many tissues, and both compounds are metabolized in part by the liver. Neither midodrine nor desglymidodrine is a substrate for monoamine oxidase.
Renal elimination of midodrine is insignificant. The renal clearance of desglymidodrine is of the order of 385 mL/minute, most, about 80%, by active renal secretion. The actual mechanism of active secretion has not been studied, but it is possible that it occurs by the base-secreting pathway responsible for the secretion of several other drugs that are bases (see also PRECAUTIONS: Potential for Drug Interactions).
Clinical Studies
Midodrine has been studied in 3 principal controlled trials, one of 3-weeks duration and 2 of 1 to 2 days duration. All studies were randomized, double-blind and parallel-design trials in patients with orthostatic hypotension of any etiology and supine-to-standing fall of systolic blood pressure of at least 15 mmHg accompanied by at least moderate dizziness/light-headedness. Patients with preexisting sustained supine hypertension above 180/110 mmHg were routinely excluded. In a 3-week study in 170 patients, most previously untreated with midodrine, the midodrine-treated patients (10 mg t.i.d., with the last dose not later than 6 P.M.) had significantly higher (by about 20 mmHg) 1-minute standing systolic pressure 1 hour after dosing (blood pressures were not measured at other times) for all 3 weeks. After week 1, midodrine-treated patients had small improvements in dizziness/light-headedness/unsteadiness scores and global evaluations, but these effects were made difficult to interpret by a high early drop-out rate (about 25% vs. 5% on placebo). Supine and sitting blood pressure rose 16/8 mmHg and 20/10 mmHg, respectively, on average.
In a 2-day study, after open-label midodrine, known midodrine responders received midodrine 10 mg or placebo at 0, 3, and 6 hours. One-minute standing systolic blood pressures were increased 1 hour after each dose by about 15 mmHg and 3 hours after each dose by about 12 mmHg; 3-minute standing pressures were increased also at 1, but not 3, hours after dosing. There were increases in standing time seen intermittently 1 hour after dosing, but not at 3 hours.
In a 1-day, dose-response trial, single doses of 0 mg, 2.5 mg, 10 mg, and 20 mg of midodrine were given to 25 patients. The 10 mg and 20 mg doses produced increases in standing 1-minute systolic pressure of about 30 mmHg at 1 hour; the increase was sustained in part for 2 hours after 10 mg and 4 hours after 20 mg. Supine systolic pressure was ≥ 200 mmHg in 22% of patients on 10 mg and 45% of patients on 20 mg; elevated pressures often lasted 6 hours or more.
INDICATIONS AND USAGE
Midodrine hydrochloride tablets are indicated for the treatment of symptomatic orthostatic hypotension (OH). Because midodrine hydrochloride tablets can cause marked elevation of supine blood pressure (BP > 200 mmHg systolic), it should be used in patients whose lives are considerably impaired despite standard clinical care, including non-pharmacologic treatment (such as support stockings), fluid expansion, and lifestyle alterations. The indication is based on midodrine hydrochloride tablets' effect on increases in 1-minute standing systolic blood pressure, a surrogate marker considered likely to correspond to a clinical benefit. At present, however, clinical benefits of midodrine hydrochloride tablets, principally improved ability to perform life activities, have not been established. Further clinical trials are underway to verify and describe the clinical benefits of midodrine hydrochloride tablets. After initiation of treatment, midodrine hydrochloride tablets should be continued only for patients who report significant symptomatic improvement.
CONTRAINDICATIONS
Midodrine hydrochloride tablets are contraindicated in patients with severe organic heart disease, acute renal disease, urinary retention, pheochromocytoma or thyrotoxicosis. Midodrine hydrochloride tablets should not be used in patients with persistent and excessive supine hypertension.
WARNINGS
Supine Hypertension
The most potentially serious adverse reaction associated with midodrine hydrochloride tablets therapy is marked elevation of supine arterial blood pressure (supine hypertension). Systolic pressures of about 200 mmHg were seen overall in about 13.4% of patients given 10 mg of midodrine hydrochloride tablets. Systolic elevations of this degree were most likely to be observed in patients with relatively elevated pre-treatment systolic blood pressures (mean 170 mmHg). There is no experience in patients with initial supine systolic pressure above 180 mmHg, as those patients were excluded from the clinical trials. Use of midodrine hydrochloride tablets in such patients is not recommended. Sitting blood pressures were also elevated by midodrine hydrochloride tablets therapy. It is essential to monitor supine and sitting blood pressures in patients maintained on midodrine.Uncontrolled hypertension increases the risk of cardiovascular events, particularly stroke.
PRECAUTIONS
General
The potential for supine and sitting hypertension should be evaluated at the beginning of midodrine hydrochloride tablets therapy. Supine hypertension can often be controlled by preventing the patient from becoming fully supine, i.e., sleeping with the head of the bed elevated. The patient should be cautioned to report symptoms of supine hypertension immediately. Symptoms may include cardiac awareness, pounding in the ears, headache, blurred vision, etc. The patient should be advised to discontinue the medication immediately if supine hypertension persists.
Blood pressure should be monitored carefully when midodrine hydrochloride tablets are used concomitantly with other agents that cause vasoconstriction, such as phenylephrine, ephedrine, dihydroergotamine, phenylpropanolamine, or pseudoephedrine.
A slight slowing of the heart rate may occur after administration of midodrine hydrochloride tablets, primarily due to vagal reflex. Caution should be exercised when midodrine hydrochloride tablets are used concomitantly with cardiac glycosides (such as digitalis), psychopharmacologic agents, beta blockers or other agents that directly or indirectly reduce heart rate. Patients who experience any signs or symptoms suggesting bradycardia (pulse slowing, increased dizziness, syncope, cardiac awareness) should be advised to discontinue midodrine hydrochloride tablets and should be reevaluated.
Midodrine hydrochloride tablets should be used cautiously in patients with urinary retention problems, as desglymidodrine acts on the alpha-adrenergic receptors of the bladder neck.
Midodrine hydrochloride tablets should be used with caution in orthostatic hypotensive patients who are also diabetic, as well as those with a history of visual problems who are also taking fludrocortisone acetate, which is known to cause an increase in intraocular pressure and glaucoma.
Midodrine hydrochloride tablets use has not been studied in patients with renal impairment. Because desglymidodrine is eliminated via the kidneys, and higher blood levels would be expected in such patients, midodrine hydrochloride tablets should be used with caution in patients with renal impairment, with a starting dose of 2.5 mg (see DOSAGE AND ADMINISTRATION). Renal function should be assessed prior to initial use of midodrine hydrochloride tablets.
Midodrine hydrochloride tablets use has not been studied in patients with hepatic impairment. Midodrine hydrochloride tablets should be used with caution in patients with hepatic impairment, as the liver has a role in the metabolism of midodrine.
Information for Patients
Patients should be told that certain agents in over-the-counter products, such as cold remedies and diet aids, can elevate blood pressure, and therefore, should be used cautiously with midodrine hydrochloride tablets, as they may enhance or potentiate the pressor effects of midodrine hydrochloride tablets (see Drug Interactions). Patients should also be made aware of the possibility of supine hypertension. They should be told to avoid taking their dose if they are to be supine for any length of time, i.e., they should take their last daily dose of midodrine hydrochloride tablets 3 to 4 hours before bedtime to minimize nighttime supine hypertension.
Laboratory Tests
Since desglymidodrine is eliminated by the kidneys and the liver has a role in its metabolism, evaluation of the patient should include assessment of renal and hepatic function prior to initiating therapy and subsequently, as appropriate.
Drug Interactions
When administered concomitantly with midodrine hydrochloride tablets, cardiac glycosides may enhance or precipitate bradycardia, A.V. block or arrhythmia.
The risk of hypertension increases with concomitant administration of drugs that increase blood pressure (phenylephrine, pseudoephedrine, ephedrine, dihydroergotamine, thyroid hormones, or droxidopa). Avoid concomitant use of drugs that increase blood pressure. If concomitant use cannot be avoided, monitor blood pressure closely.
Avoid use of MAO inhibitors or linezolid with midodrine.
Midodrine hydrochloride tablets has been used in patients concomitantly treated with salt-retaining steroid therapy (i.e., fludrocortisone acetate), with or without salt supplementation. The potential for supine hypertension should be carefully monitored in these patients and may be minimized by either reducing the dose of fludrocortisone acetate or decreasing the salt intake prior to initiation of treatment with midodrine hydrochloride tablets. Alpha-adrenergic blocking agents, such as prazosin, terazosin, and doxazosin, can antagonize the effects of midodrine hydrochloride tablets.
Potential for Drug Interactions
It appears possible, although there is no supporting experimental evidence, that the high renal clearance of desglymidodrine (a base) is due to active tubular secretion by the base-secreting system also responsible for the secretion of such drugs as metformin, cimetidine, ranitidine, procainamide, triamterene, flecainide, and quinidine. Thus there may be a potential for drug-drug interactions with these drugs.
Carcinogenesis, Mutagenesis, Impairment of Fertility
Long-term studies have been conducted in rats and mice at dosages of 3 to 4 times the maximum recommended daily human dose on a mg/m 2 basis, with no indication of carcinogenic effects related to midodrine hydrochloride tablets. Studies investigating the mutagenic potential of midodrine hydrochloride tablets revealed no evidence of mutagenicity. Other than the dominant lethal assay in male mice, where no impairment of fertility was observed, there have been no studies on the effects of midodrine hydrochloride tablets on fertility.
Pregnancy
Pregnancy Category C
Midodrine hydrochloride tablets increased the rate of embryo resorption, reduced fetal body weight in rats and rabbits, and decreased fetal survival in rabbits when given in doses 13 (rat) and 7 (rabbit) times the maximum human dose based on body surface area (mg/m 2). There are no adequate and well-controlled studies in pregnant women. Midodrine hydrochloride tablets should be used during pregnancy only if the potential benefit justifies the potential risk to the fetus. No teratogenic effects have been observed in studies in rats and rabbits.
ADVERSE REACTIONS
The most frequent adverse reactions seen in controlled trials were supine and sitting hypertension; paresthesia and pruritus, mainly of the scalp; goosebumps; chills; urinary urge; urinary retention and urinary frequency.
The frequency of these events in a 3-week placebo-controlled trial is shown in the following table:
| Placebo
n = 88 | Midodrine
n = 82 |
|||
|---|---|---|---|---|
| Event | # of reports | % of patients | # of reports | % of patients |
|
||||
|
Total # of reports |
22 |
77 | ||
|
Paresthesia * |
4 |
4.5 |
15 |
18.3 |
|
Piloerection |
0 |
0 |
11 |
13.4 |
|
Dysuria † |
0 |
0 |
11 |
13.4 |
|
Pruritus ‡ |
2 |
2.3 |
10 |
12.2 |
|
Supine hypertension § |
0 |
0 |
6 |
7.3 |
|
Chills |
0 |
0 |
4 |
4.9 |
|
Pain ¶ |
0 |
0 |
4 |
4.9 |
|
Rash |
1 |
1.1 |
2 |
2.4 |
Less frequent adverse reactions were headache; feeling of pressure/fullness in the head; vasodilation/flushing face; confusion/thinking abnormality; dry mouth; nervousness/anxiety and rash. Other adverse reactions that occurred rarely were visual field defect; dizziness; skin hyperesthesia; insomnia; somnolence; erythema multiforme; canker sore; dry skin; dysuria; impaired urination; asthenia; backache; pyrosis; nausea; gastrointestinal distress; flatulence and leg cramps.
The most potentially serious adverse reaction associated with midodrine hydrochloride tablets therapy is supine hypertension. The feelings of paresthesia, pruritus, piloerection and chills are pilomotor reactions associated with the action of midodrine on the alpha-adrenergic receptors of the hair follicles. Feelings of urinary urgency, retention and frequency are associated with the action of midodrine on the alpha-receptors of the bladder neck.
OVERDOSAGE
Symptoms of overdose could include hypertension, piloerection (goosebumps), a sensation of coldness and urinary retention. There are 2 reported cases of overdosage with midodrine hydrochloride tablets, both in young males. One patient ingested midodrine hydrochloride drops, 250 mg, experienced systolic blood pressure of greater than 200 mmHg, was treated with an IV injection of 20 mg of phentolamine, and was discharged the same night without any complaints. The other patient ingested 205 mg of midodrine hydrochloride (41 5 mg tablets), and was found lethargic and unable to talk, unresponsive to voice but responsive to painful stimuli, hypertensive and bradycardic. Gastric lavage was performed, and the patient recovered fully by the next day without sequelae.
The single doses that would be associated with symptoms of overdosage or would be potentially life threatening are unknown. The oral LD 50 is approximately 30 to 50 mg/kg in rats, 675 mg/kg in mice, and 125 to 160 mg/kg in dogs.
Desglymidodrine is dialyzable.
Recommended general treatment, based on the pharmacology of the drug, includes induced emesis and administration of alpha-sympatholytic drugs (e.g., phentolamine).
DOSAGE AND ADMINISTRATION
The recommended dose of midodrine hydrochloride tablets is 10 mg, 3 times daily. Dosing should take place during the daytime hours when the patient needs to be upright, pursuing the activities of daily life. A suggested dosing schedule of approximately 4-hour intervals is as follows: shortly before or upon arising in the morning, midday, and late afternoon (not later than 6 P.M.). Doses may be given in 3-hour intervals, if required, to control symptoms, but not more frequently. Single doses as high as 20 mg have been given to patients, but severe and persistent systolic supine hypertension occurs at a high rate (about 45%) at this dose. In order to reduce the potential for supine hypertension during sleep, midodrine hydrochloride tablets should not be given after the evening meal or less than 4 hours before bedtime. Total daily doses greater than 30 mg have been tolerated by some patients, but their safety and usefulness have not been studied systematically or established. Because of the risk of supine hypertension, midodrine hydrochloride tablets should be continued only in patients who appear to attain symptomatic improvement during initial treatment.
The supine and standing blood pressure should be monitored regularly, and the administration of midodrine hydrochloride tablets should be stopped if supine blood pressure increases excessively.
Because desglymidodrine is excreted renally, dosing in patients with abnormal renal function should be cautious; although this has not been systematically studied, it is recommended that treatment of these patients be initiated using 2.5 mg doses.
Dosing in children has not been adequately studied.
Blood levels of midodrine and desglymidodrine were similar when comparing levels in patients 65 or older vs. younger than 65 and when comparing males vs. females, suggesting dose modifications for these groups are not necessary.
HOW SUPPLIED
Midodrine Hydrochloride Tablets, USP are available containing 5 mg of midodrine hydrochloride, USP.
The 5 mg are tablets round, white scored tablet with C/150 on the upper side; and plain on the lower side. They are available as follows:
Boxes of 10 x 10 UD 100, NDC 63739-145-10
Store at 20° to 25°C (68° to 77°F). [See USP Controlled Room Temperature.]
Dispense in a tight, light-resistant container as defined in the USP using a child-resistant closure.
Manufactured by:
Cerovene, LLC
Valley Cottage, NY 10989
Distributed by:
McKesson Corporation dba SKY Packaging
Memphis, TN 38141
21991
Rev.: August 2022
