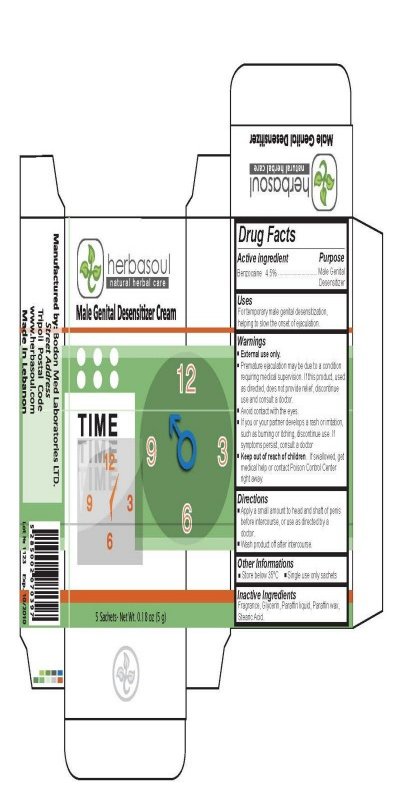
Active Ingredient Purpose
Benzocaine 4.5 percent .......................................................................Male Genital
Desensitizer
Warnings
External use only.
Premature ejaculation may be due to a condition requiring medical supervision.
If this product, used as directed, does not provide relief, discontinue use. If symptoms persist,consult a doctor.
Keep out of reach of children.
If swallowed, get medical help or contact Poison Control Center right away.
Directions
Apply a small amount to head and shaft of penis before intercourse,
or use as directed by a doctor.
Wash product off after intercourse.
Manufactured By: Bodon Med Laboratories LTD.
Street Address
Tripoli Postal Code
www.herbasoul.com
Made in Lebanon