FULL PRESCRIBING INFORMATION
1 INDICATIONS AND USAGE
Fluticasone propionate ointment, USP 0.005% is a corticosteroid indicated for the relief of the inflammatory and pruritic manifestations of corticosteroid-responsive dermatoses in adult patients.
2 DOSAGE AND ADMINISTRATION
Apply a thin film of fluticasone propionate ointment, 0.005% to the affected skin areas twice daily. Rub in gently.
Avoid use with occlusive dressing.
Fluticasone propionate ointment, 0.005% is for topical use only; it is not for ophthalmic, oral or intravaginal use.
3 DOSAGE FORMS AND STRENGTHS
Ointment, 0.005%. Each gram of fluticasone propionate ointment, 0.005% contains 0.05 mg fluticasone propionate in a white to off-white translucent ointment base. Fluticasone propionate ointment, 0.005% is supplied in 15 g, 30 g, and 60 g tubes.
4 CONTRAINDICATIONS
Fluticasone propionate ointment, 0.005% is contraindicated in those patients with a history of hypersensitivity to any of the components in the preparation.
5 WARNINGS AND PRECAUTIONS
5.1 Hypothalamic-Pituitary-Adrenal (HPA) Axis Suppression and Other Adverse Endocrine Effects
Topical corticosteroids, including fluticasone propionate ointment, 0.005%, can produce reversible HPA axis suppression with the potential for clinical glucocorticoid insufficiency. Factors that predispose to HPA axis suppression include large treatment surface areas, prolonged use, use under occlusion, altered skin barrier, liver failure, and young age. Cushing’s syndrome, hyperglycemia, and unmasking of latent diabetes mellitus can also result from systemic absorption of topical corticosteroids.
If HPA axis suppression is suspected, gradually withdraw the drug, reduce the frequency of application, or substitute with a less potent corticosteroid. An ACTH stimulation test may be helpful in evaluating patients for HPA axis suppression.
Pediatric patients may be at greater risk of HPA axis suppression due to their higher skin surface area to body mass ratios [ see Usein Specific Populations ( 8.4) ].
HPA axis suppression may occur during or after withdrawal of treatment. If HPA axis suppression is suspected, gradually withdraw the drug, reduce the frequency of application, or substitute with a less potent corticosteroid. Evaluation of HPA axis suppression may be done by using the cosyntropin stimulation test.
5.2 Local Adverse Reactions
Fluticasone propionate ointment, 0.005% may cause local adverse reactions, including skin atrophy [ see Adverse Reactions( 6.1, 6.2) ]. The risk is greater with use under occlusion.
5.3 Allergic Contact Dermatitis
Allergic contact dermatitis with topical corticosteroids is usually diagnosed by observing failure to heal rather than noting a clinical exacerbation. Such an observation can be corroborated with appropriate diagnostic patch testing. Discontinue fluticasone propionate ointment, 0.005% if appropriate.
6 ADVERSE REACTIONS
The following adverse reactions are discussed in greater detail in other sections of the labeling:
• HPA Axis Suppression and Other Adverse Endocrine Effects [
seeWarnings and Precautions (
5.1)
]
• Local Adverse Reactions [
see Warnings and Precautions (
5.2)
]
• Concomitant Skin Infections [
see Warnings and Precautions(
5.3)
]
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
In controlled clinical trials, the total incidence of adverse reactions associated with the use of fluticasone propionate ointment, 0.005% was approximately 4%. These adverse reactions were usually mild, self-limiting, and consisted primarily of pruritus, burning, hypertrichosis, increased erythema, urticaria, irritation, and lightheadedness. Each of these events occurred individually in less than 1% of patients.
6.2 Postmarketing Experience
The following local adverse reactions have been identified during post-approval use of fluticasone propionate ointment, 0.005%: acneiform dermatitis, edema, rash, hypoaesthesia, pustular psoriasis, skin atrophy.
The following systemic adverse reactions have been identified during post-approval use of fluticasone propionate cream and fluticasone propionate ointment, 0.005%: immunosuppression/ Pneumocystis jiroveciipneumonia/leukopenia/thrombocytopenia; hyperglycemia/glycosuria; Cushing syndrome; generalized body edema/blurred vision; and acute urticarial reaction (edema, urticaria, pruritus, and throat swelling).
The following local adverse reactions have also been reported with the use of topical corticosteroids: telangiectasia, striae, dryness, folliculitis, hypopigmentation, perioral dermatitis, allergic contact dermatitis, secondary infection, and miliaria.
Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Pregnancy Category C
There are no adequate and well-controlled studies in pregnant women. Therefore, fluticasone propionate ointment, 0.005% should be used during pregnancy only if the potential benefit justifies the potential risk to the fetus.
Systemic embryofetal development studies were conducted in mice, rats and rabbits.
Subcutaneous doses of 15, 45 and 150 μg/kg/day of fluticasone propionate were administered to pregnant female mice from gestation days 6 to 15. A teratogenic effect characteristic of corticosteroids (cleft palate) was noted after administration of 45 and 150 μg/kg/day (less than the MRHD in adults based on body surface area comparisons) in this study. No treatment related effects on embryofetal toxicity or teratogenicity were noted at 15 μg/kg/day (less than the MRHD in adults based on body surface area comparisons).
Subcutaneous doses of 10, 30 and 100 μg/kg/day of fluticasone propionate were administered to pregnant female rats in two embryofetal development studies (one study administered fluticasone propionate from gestation days 6 to 15 and the other study from gestation days 7 to 17). In the presence of maternal toxicity, fetal effects noted at 100 μg/kg/day (less than the MRHD in adults based on body surface area comparisons) included decreased fetal weights, omphalocele, cleft palate, and retarded skeletal ossification. No treatment related effects on embryofetal toxicity or teratogenicity were noted at 10 μg/kg/day (less than the MRHD in adults based on body surface area comparisons).
Subcutaneous doses of 0.08, 0.57 and 4 μg/kg/day of fluticasone propionate were administered to pregnant female rabbits from gestation days 6 to 18. Fetal effects noted at 4 μg/kg/day (less than the MRHD in adults based on body surface area comparisons) included decreased fetal weights, cleft palate and retarded skeletal ossification. No treatment related effects on embryofetal toxicity or teratogenicity were noted at 0.57 μg/kg/day (less than the MRHD in adults based on body surface area comparisons).
Oral doses of 3, 30 and 300 μg/kg/day fluticasone propionate were administered to pregnant female rabbits from gestation days 8 to 20. No fetal or teratogenic effects were noted at oral doses up to 300 μg/kg/day (less than the MRHD in adults based on body surface area comparisons) in this study. However, no fluticasone propionate was detected in the plasma in this study, consistent with the established low bioavailability following oral administration.
Fluticasone propionate crossed the placenta following administration of a subcutaneous or an oral dose of 100 μg/kg tritiated fluticasone propionate to pregnant rats.
8.3 Nursing Mothers
Systemically administered corticosteroids appear in human milk and can suppress growth, interfere with endogenous corticosteroid production, or cause other untoward effects. It is not known whether topical administration of corticosteroids could result in sufficient systemic absorption to produce detectable quantities in human milk. Because many drugs are excreted in human milk, caution should be exercised when fluticasone propionate ointment, 0.005% is administered to a nursing woman.
8.4 Pediatric Use
The safety and effectiveness of fluticasone propionate ointment, 0.005% have not been established in pediatric patients. Use of fluticasone propionate ointment, 0.005% in pediatric patients is not recommended.
Because of a higher ratio of skin surface area to body mass, pediatric patients are at a greater risk than adults of systemic effects when treated with topical drugs. They are, therefore, also at greater risk of HPA axis suppression and adrenal insufficiency upon the use of topical corticosteroids [ see Warnings andPrecautions ( 5.1) ].
In a trial of 35 pediatric subjects treated with fluticasone propionate ointment, 0.005% for atopic dermatitis over at least 35% of body surface area, subnormal adrenal function was observed with cosyntropin stimulation testing at the end of 3 to 4 weeks of treatment in 4 subjects who had normal testing prior to treatment. It is not known if these subjects had recovery of adrenal function because follow-up testing was not performed. The decreased responsiveness of cosyntropin testing was not correlated to age of subject, amount of fluticasone propionate ointment used, or serum levels of fluticasone propionate.
In the above trial, telangiectasia on the face was noted in one subject on the eighth day of a 4-week treatment period. Facial use was discontinued and the telangiectasia resolved.
HPA axis suppression, Cushing’s syndrome, linear growth retardation, delayed weight gain, and intracranial hypertension have been reported in pediatric patients receiving topical corticosteroids.
8.5 Geriatric Use
A limited number of patients above 65 years of age (n = 203) have been treated with fluticasone propionate ointment, 0.005% in US and non-US clinical trials. No overall differences in safety or effectiveness were observed between these subjects and younger subjects, and other reported clinical experience has not identified differences in responses between the elderly and younger patients. However, greater sensitivity of some older individuals cannot be ruled out.
11 DESCRIPTION
Fluticasone propionate ointment, USP 0.005% contains fluticasone propionate [ S-Fluoromethyl 6α, 9α-difluoro-11β-hydroxy-16α-methyl-3-oxo-17α-propionyloxyandrosta-1,4-diene-17β-carbothioate], a synthetic fluorinated corticosteroid, for topical use.
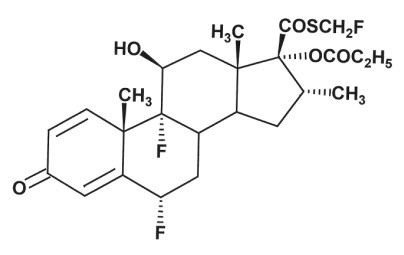
Chemically, fluticasone propionate is C 25H 31F 3O 5S. It has the following structural formula:
Fluticasone propionate has a molecular weight of 500.6. It is a white to off-white powder and is insoluble in water.
Each gram of fluticasone propionate ointment contains fluticasone propionate 0.05 mg in a white to off-white translucent ointment base of mineral oil, microcrystalline wax, propylene glycol, and sorbitan sesquioleate.
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
Corticosteroids play a role in cellular signaling, immune function, inflammation, and protein regulation; however, the precise mechanism of action of fluticasone propionate ointment, 0.005% in corticosteroid-responsive dermatoses is unknown.
12.2 Pharmacodynamics
Vasoconstrictor Assay
Studies performed with fluticasone propionate ointment, 0.005% indicate that it is in the medium range of potency as demonstrated in vasoconstrictor trials in healthy subjects when compared with other topical corticosteroids. However, similar blanching scores do not necessarily imply therapeutic equivalence.
12.3 Pharmacokinetics
Absorption
The extent of percutaneous absorption of topical corticosteroids is determined by many factors, including the vehicle and the integrity of the epidermal barrier. Occlusive dressing enhances penetration. Topical corticosteroids can be absorbed from normal intact skin. Inflammation and/or other disease processes in the skin increase percutaneous absorption.
In a study of 6 healthy subjects applying 25 g of fluticasone propionate ointment 0.005% twice daily to the trunk and legs for up to 5 days under occlusion, plasma levels of fluticasone ranged from 0.08 to 0.22 ng/mL.
Distribution
The percentage of fluticasone propionate bound to human plasma proteins averaged 91%. Fluticasone propionate is weakly and reversibly bound to erythrocytes. Fluticasone propionate is not significantly bound to human transcortin.
Metabolism
No metabolites of fluticasone propionate were detected in an in vitro study of radiolabeled fluticasone propionate incubated in human skin homogenate.
Fluticasone propionate is metabolized in the liver by cytochrome P450 3A4-mediated hydrolysis of the 5-fluoromethyl carbothioate grouping. This transformation occurs in 1 metabolic step to produce the inactive 17-β-carboxylic acid metabolite, the only known metabolite detected in man. This metabolite has approximately 2,000 times less affinity than the parent drug for the glucocorticoid receptor of human lung cytosol in vitro and negligible pharmacological activity in animal studies. Other metabolites detected in vitro using cultured human hepatoma cells have not been detected in man.
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
In an oral (gavage) mouse carcinogenicity study, doses of 0.1, 0.3 and 1 mg/kg/day fluticasone propionate were administered to mice for 18 months. Fluticasone propionate demonstrated no tumorigenic potential at oral doses up to 1 mg/kg/day (less than the MRHD in adults based on body surface area comparisons) in this study.
In a dermal mouse carcinogenicity study, 0.05% fluticasone propionate ointment (40 μl) was topically administered for 1, 3 or 7 days/week for 80 weeks. Fluticasone propionate demonstrated no tumorigenic potential at dermal doses up to 6.7 μg/kg/day (less than the MRHD in adults based on body surface area comparisons) in this study.
Fluticasone propionate revealed no evidence of mutagenic or clastogenic potential based on the results of five in vitro genotoxicity tests (Ames assay, E. coli fluctuation test, S. cerevisiae gene conversion test, Chinese hamster ovary cell chromosome aberration assay and human lymphocyte chromosome aberration assay) and one in vivo genotoxicity test (mouse micronucleus assay).
No evidence of impairment of fertility or effect on mating performance was observed in a fertility and general reproductive performance study conducted in male and female rats at subcutaneous doses up to 50 μg/kg/day (less than the MRHD in adults based on body surface area comparisons).
16 HOW SUPPLIED/STORAGE AND HANDLING
Fluticasone Propionate Ointment, USP 0.005% is a white to off-white translucent ointment supplied as follows:
15 g tubes (NDC 0713-0632-15)
30 g tubes (NDC 0713-0632-31)
60 g tubes (NDC 0713-0632-60)
Store between 2° and 30°C (36° and 86°F).
17 PATIENT COUNSELING INFORMATION
Advise the patient to read the FDA-approved patient labeling (Patient Information).
Advise the patient:
- Avoid contact with the eyes.
- Do not bandage the treated skin area, or cover or wrap it to cause occlusion unless directed by the healthcare provider.
- Report any signs of local adverse reaction to their healthcare provider.
- Do not use on the face, underarms, or groin areas unless directed by the healthcare provider.
Distributed by:
Cosette Pharmaceuticals, Inc.
South Plainfield, NJ 07080
8-0632CPLNC2
Rev. 10/2022 VC7680
PATIENT INFORMATION
Fluticasone Propionate Ointment, USP 0.005%
Important: Fluticasone propionate ointment, 0.005% is for use on skin only (topical). Do not get fluticasone propionate ointment, 0.005% near or in your eyes, mouth, or vagina.
Read this Patient Information before you start using fluticasone propionate ointment, 0.005% and each time you get a refill.
There may be new information. This information does not take the place of talking to your healthcare provider about your medical condition or your treatment.
What is fluticasone propionate ointment, 0.005%?
Fluticasone propionate ointment, 0.005% is a prescription corticosteroid medicine used on the skin (topical) for the relief of inflammation and itching caused by certain skin conditions in adults.
It is not known if fluticasone propionate ointment, 0.005% is safe and effective in children. Fluticasone propionate ointment, 0.005% is not recommended for use in children.
Before using fluticasone propionate ointment, 0.005%, tell your healthcare provider about all of your medical conditions, including if you:
- have an allergy to any of the ingredients in fluticasone propionate ointment, 0.005%
- have a skin infection at the site to be treated. You may also need medicine to treat the skin infection.
- have adrenal gland problems
- have liver problems
- have diabetes
- have thinning skin (atrophy) at the site to be treated.
- are pregnant or plant to become pregnant. it is not known if fluticasone propionate ointment, 0.005% will harm your unborn baby.
- are breastfeeding or plan to breastfeed. It is not known if fluticasone propionate ointment, 0.005% can pass into your breast milk and harm your baby.
Tell your healthcare provider about all the medicines you take, including prescription and over-the-counter medicines, vitamins, and herbal supplements. Especially tell your healthcare provider if you take other corticosteroid medicines by mouth or use other products on your skin that contain corticosteroids.
How should I use fluticasone propionate ointment, 0.005%?
- Use fluticasone propionate ointment, 0.005% exactly as your healthcare provider tells you to use it.
- Apply a thin film of fluticasone propionate ointment, 0.005% to the affected area 2 times each day. Gently rub into your skin.
- Do not bandage, cover, or wrap the treated area unless your healthcare provider tells you to.
- Do not use fluticasone propionate ointment, 0.005% on your face, groin, underarms (armpits), unless your healthcare provider tells you to.
- Wash your hands after applying fluticasone propionate ointment, 0.005%, unless your hands are being treated.
- Tell your healthcare provider if your symptoms get worse with fluticasone propionate ointment, 0.005% or if your symptoms do not improve after 2 weeks of treatment.
What are the possible side effects of fluticasone propionate ointment, 0.005%?
Fluticasone propionate ointment, 0.005% may cause serious side effects, including:
- Fluticasone propionate ointment, 0.005% can pass through your skin and maycause adrenal gland problems. This is more likely to happen if you use fluticasone propionate ointment, 0.005% for too long, use it over a large treatment area, use it with other topical medicines that contain corticosteroids, cover the treated area, or have liver failure. Your healthcare provider may do blood tests to check your adrenal gland function during and after treatment with fluticasone propionate ointment, 0.005%.
- Skin problems, including skin reactions or thinning of your skin (atrophy), skin infections, and allergic reactions(allergic contact dermatitis) at the treatment site. Tell your healthcare provider if you get any skin reactions such as pain, tenderness, swelling, or healing problems.
The most common side effects of fluticasone propionate ointment, 0.005%include itching, burning, excessive hair growth, skin redness, hives, and lightheadedness.
These are not all the possible side effects of fluticasone propionate ointment, 0.005%. Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088.
How should I store fluticasone propionate ointment, 0.005%?
- Store fluticasone propionate ointment, 0.005% between 36°F to 86°F (2°C to 30°C).
Keep fluticasone propionate ointment, 0.005% and all medicines out of the reach of children.
General information about the safe and effective use of fluticasone propionate ointment, 0.005%.
Medicines are sometimes prescribed for purposes other than those listed in a Patient Information leaflet. Do not use fluticasone propionate ointment, 0.005% for a condition for which it was not prescribed. Do not give fluticasone propionate ointment, 0.005% to other people, even if they have the same symptoms you have. It may harm them. You can ask your pharmacist or healthcare provider for information about fluticasone propionate ointment, 0.005% that is written for health professionals.
What are the ingredients in fluticasone propionate ointment, 0.005%?
Active ingredient:fluticasone propionate
Inactive ingredients:base of mineral oil, microcrystalline wax, propylene glycol, and sorbitan sesquioleate.
Distributed by:
Cosette Pharmaceuticals, Inc.
South Plainfield, NJ 07080
8-0632CPLNC2
Rev. 10/2022 VC7680
This Patient Information has been approved by the U.S. Food and Drug Administration.
Rev. 10/2022
PRINCIPAL DISPLAY PANEL
NDC 0713-0632-15
Fluticasone Propionate Ointment,USP 0.005%
15 g
Rx only
FOR TOPICAL USE ONLY. NOT FOR OPHTHALMIC USE.
Cosette Pharmaceuticals, Inc.

NDC 0713-0632-31
Fluticasone Propionate Ointment,USP 0.005%
30 g
Rx only
FOR TOPICAL USE ONLY. NOT FOR OPHTHALMIC USE.
Cosette Pharmaceuticals, Inc.

NDC 0713-0632-60
Fluticasone Propionate Ointment,USP 0.005%
60 g
Rx only
FOR TOPICAL USE ONLY. NOT FOR OPHTHALMIC USE.
Cosette Pharmaceuticals, Inc.
