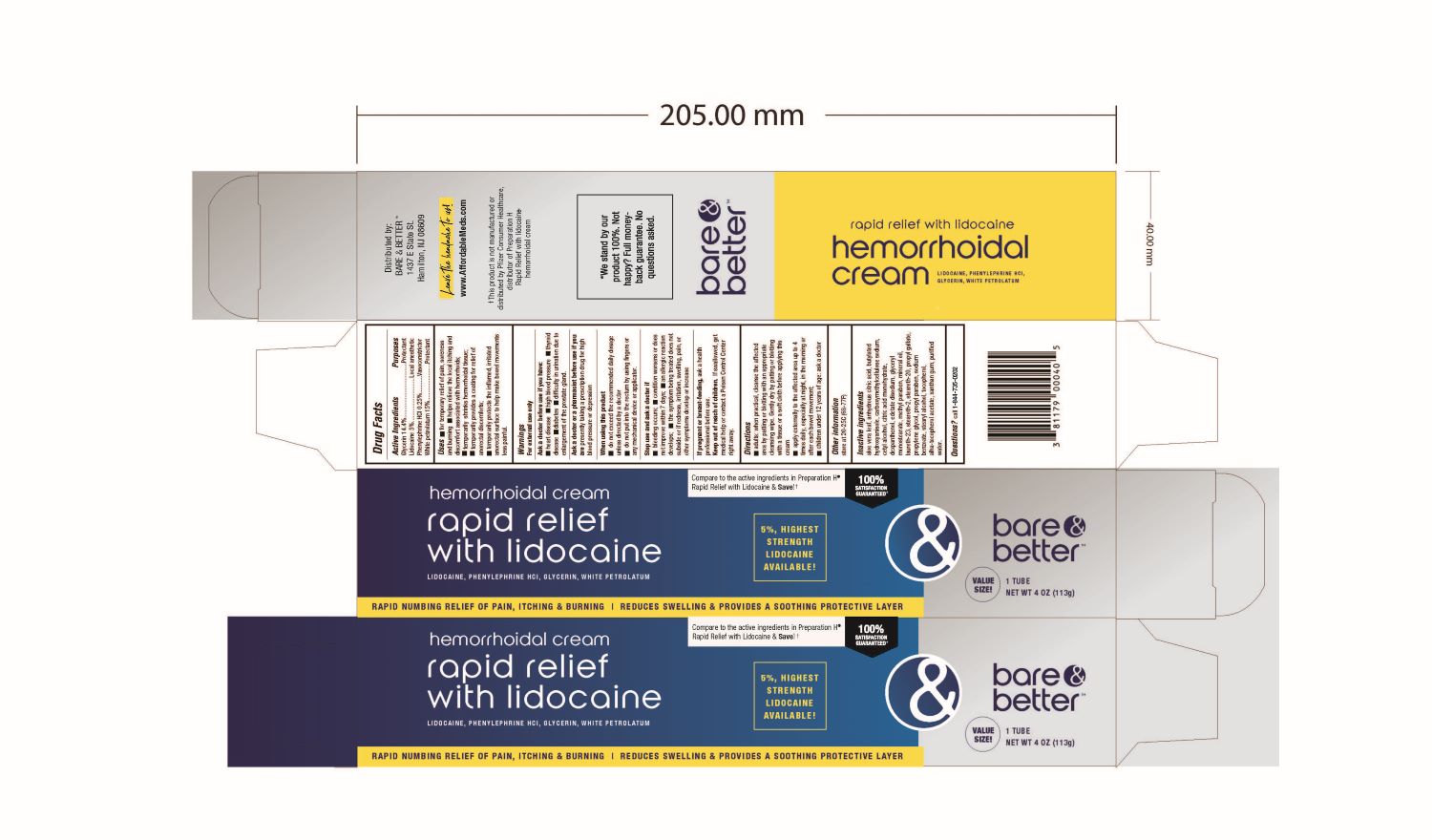
Uses:
- For temporary relief of pain, soreness and burning
- Helps relieve the local itching and discomfort associated with hemorrhoids
- Temporarily shrinks hemorrhoidal tissue
- Temporarily provides a coating for relief of anorectal discomforts
- Temporarily protects the inflamed irritated anorectal surface to help make bowel movements less painful
Warnings:
- Ask a doctor before use if you have
- Ask a doctor or pharmacist before use if you are presently taking a prescription drug for high blood pressure or depression.
Directions:
- Adults: when practical, cleanse the affected area by patting or blotting with an appropriate cleansing wipe. Gently dry by patting or blotting with a tissue or a soft cloth before applying this cream.
- Apply externally to the affected area up to 4 times daily, especially at night, in the morning or after each bowel movement
- Children under 12 years of age: ask a doctor
Keep out of reach of children. If swallowed, get medical help or contact a Poison Control Center right
away.
Inactive Ingredients:
aloe vera leaf, anhydrous citric acid, butylated hydroxyanisole, carboxymethylcellulose sodium, cetyl alcohol, citric acid monohydrate, dexpanthenol, edetate disodium, glyceryl monosterate, methylparaben, mineral oil, laureth-23, steareth-2, steareth-20, propyl gallate, propylene glycol, propylparaben, sodium benzoate, tocopherol, alpha.- tocopherol acetate, xanthan gum, water