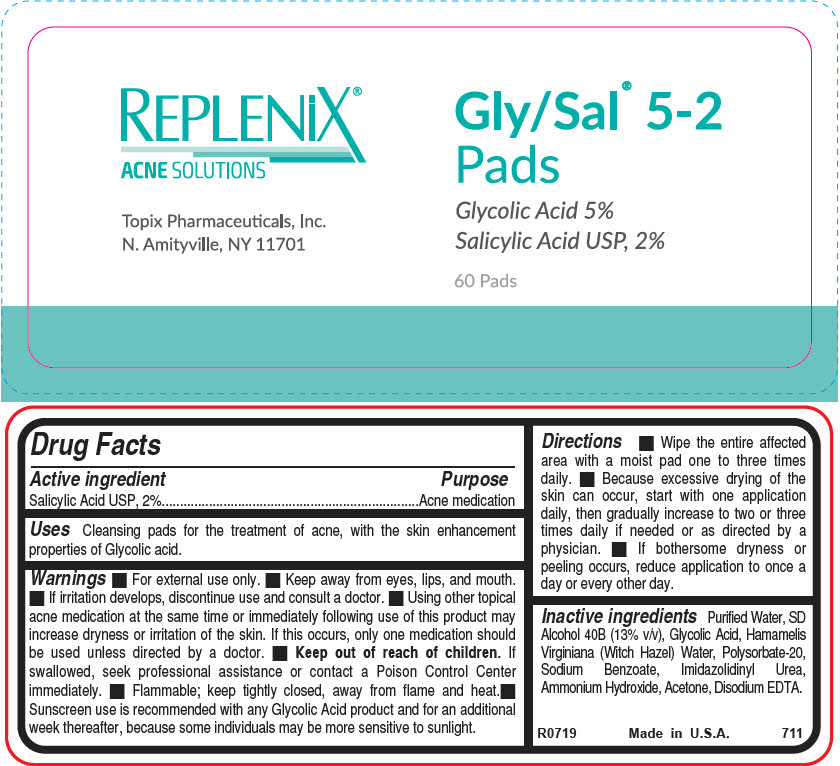
Active ingredient
Salicylic Acid USP, 2%
Uses
Cleansing pads for the treatment of acne, with the skin enhancement properties of Glycolic acid.
Warnings
- For external use only.
- Keep away from eyes, lips, and mouth.
- If irritation develops, discontinue use and consult a doctor.
- Using other topical acne medication at the same time or immediately following use of this product may increase dryness or irritation of the skin. If this occurs, only one medication should be used unless directed by a doctor.
-
Keep out of reach of children. If swallowed, seek professional assistance or contact a Poison Control Center immediately.
- Flammable; keep tightly closed, away from flame and heat.
- Sunscreen use is recommended with any Glycolic Acid product and for an additional week thereafter, because some individuals may be more sensitive to sunlight.
Directions
- Wipe the entire affected area with a moist pad one to three times daily.
- Because excessive drying of the skin can occur, start with one application daily, then gradually increase to two or three times daily if needed or as directed by a physician.
- If bothersome dryness or peeling occurs, reduce application to once a day or every other day.
Inactive ingredients
Purified Water, SD Alcohol 40B (13% v/v), Glycolic Acid, Hamamelis Virginiana (Witch Hazel) Water, Polysorbate-20, Sodium Benzoate, Imidazolidinyl Urea, Ammonium Hydroxide, Acetone, Disodium EDTA.
PRINCIPAL DISPLAY PANEL - 60 Pad Jar Label
REPLENiX®
ACNE SOLUTIONS
Topix Pharmaceuticals, Inc.
N. Amityville, NY 11701
Gly/Sal® 5-2
Pads
Glycolic Acid 5%
Salicylic Acid USP, 2%
60 Pads
Topiderm, Inc.