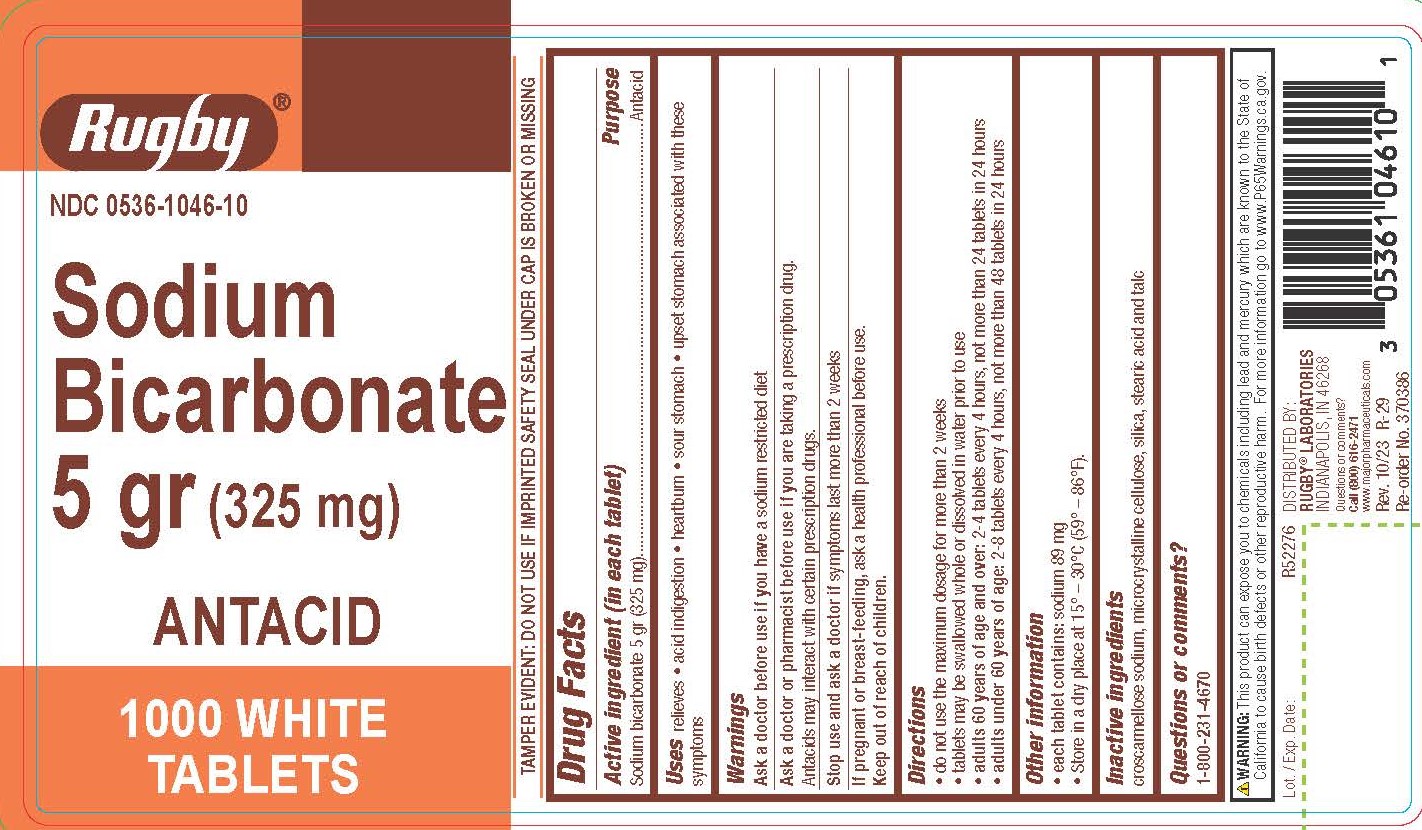
Warnings
Directions
- do not use the maximum dosage for more than 2 weeks
- tablets may be swallowed whole or dissolved in water prior to use
- adults 60 years of age and over: 2-4 tablets every 4 hours, not more than 24 tablets in 24 hours
- adults under 60 years of age: 2-8 tablets every 4 hours, not more than 48 tablets in 24 hours
Other information
- each tablet contains: sodium 89 mg
- Store in a dry place at 15º - 30ºC (59º - 86º F)
Inactive ingredients
croscarmellose sodium, microcrystalline cellulose, silica, stearic acid, and talc
TAMPER EVIDENT: DO NOT USE IF IMPRINTED SAFETY SEAL UNDER CAP IS BROKEN OR MISSING
WARNING: This product can expose you to chemicals including lead and mercury which are known to the State of
California to cause birth defects or other reproductive harm. For more information go to www.P65Warnings.ca.gov.
DISTRIBUTED BY:
RUGBY® LABORATORIES
INDIANAPOLIS, IN 46268
Questions or comments?
Call (800) 616-2471
www.majorpharmaceuticals.com
Rev. 10/23 R-29