Keep out of reach of children
Keep out of reach of children. If swallowed, get medical help or contact a Poison Control Center immediately.
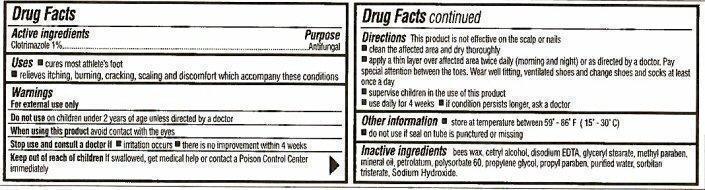
Uses
- cures most athlete's foot
- relieves itching, burning, cracking, scaling and discomfort which accompany these conditions
Warnings
For external use only
Do not use on children under 2 years of age unless directed by a doctor
When using this product avoid contact with the eyes
Stop use and consult a doctor if
- irritation occurs
- there is no improvement within 2 weeks
Directions
This product is not effective on the scalp or nails
- clean the affected area and dry thoroughly
- apply a thin layer over affected area twice daily (morning and night) or as directed by a doctor. Pay special attention between the toes. Wear well fitting, ventilated shoes and change shoes and socks at least once a day.
- supervise children in the use of this product
- use daily for 4 weeks
- if condition persists longer, ask a doctor
Other information
- store at temperature between 59° - 86° F (15° - 30° C)
- do not use if seal on tube is punctured or missing
Inactive ingredients
bees wax, cetryl alcohol, disodium EDTA, glyceryl stearate, methyl paraben, mineral oil, petrolatum, polysorbate 60, propylene glycol, propyl paraben, purified water, sorbitan tristerate, Sodium Hydroxide.
Product Label
DRUGSTORE-Rx
SMART WELLNESS
Clotrimazole USP 1%
Athlete's Foot
Antifungal Cream
Cures most major types of athlete's foot
Relieves
- Itching
- Burning
- Scaling
- Cracking
Greaseless
Odorless
Non-staining
NET WT. 1.25 OZ. (35 g)
DRUGSTORE-Rx
SMART WELLNESS delivers outstanding quality in healthcare products, at truly amazing prices. We employ the highest standard when searching the world to bring the best value to your doorstep. Use our products confidently, knowing that attention and care has gone into every item. That's smart wellness!
Distributed by: PERSONAL CARE PRODUCTS, LLC
Troy, Michigan 48084 U.S.A.
Code No. MH/DRUGS/KD-313 Made in India