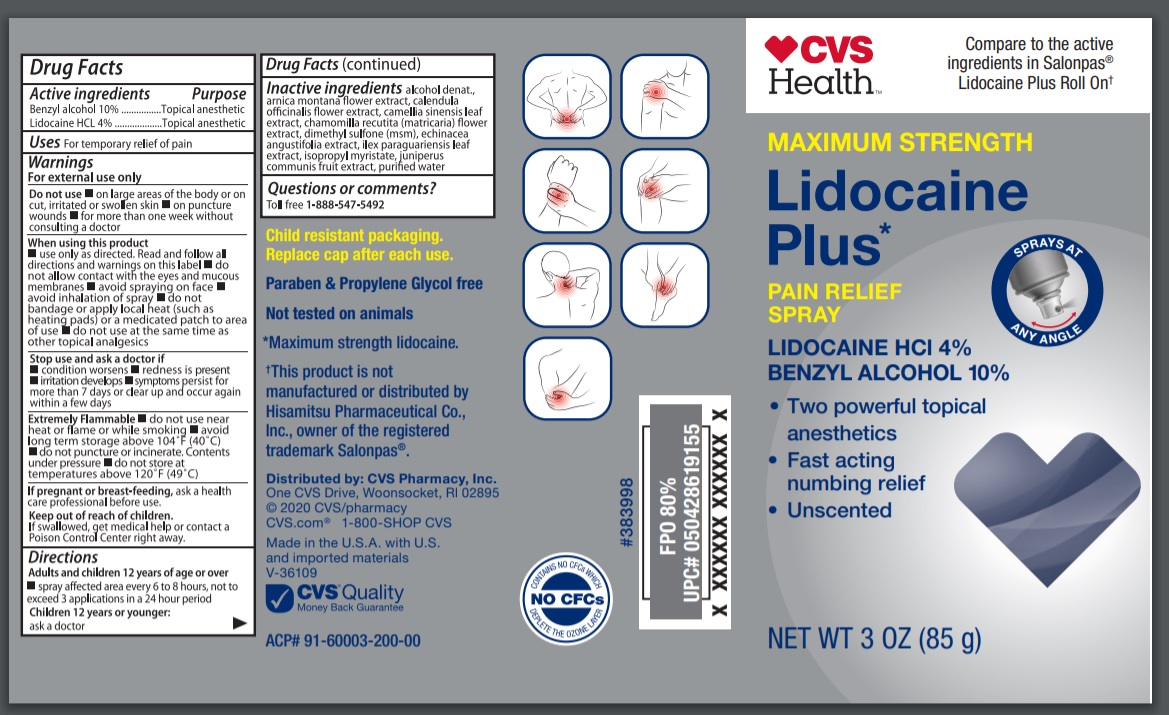
Warnings
For external use only
Flammable:
Keep away from fire or flame
-Do not use near heat or flame or while smoking.
-avoid long term storage above 40 degree Celcius.
-do not puncture or incinerate. COntents under pressure
-do not store at temperatures above 49 degree Celcius
Do not use
- on large areas of the body or cut or wounds or damaged skin
- on puncture wounds
- for more than one week without consulting a doctor
When using this product
- use only as directed
- avoid contact with the eyes, mucous membranes or rashes
- avoid spraying on face
- avoid inhalation of spray
- do not bandage or apply local heat such as heating pads or a medicated patch to area of use
- do not use at the same time as other topical analgesics
Directions
Adults and children 12 years of age and over:
- spray to affected area not more than 3 to 4 times daily
Children under 12 years of age:
consult a doctor
Other information
- Avoid storing product in direct sunlight
- Protect product from excessive moisture
- Store with lid closed tighly
Inactive ingredients
alcohol denatured, arnica montana flower extract, calendula officinalis flower extract, camellia sinensis leaf extract, chamomilla recutita (matricaria) flower extract , dimethyl sulfone (msm), Echinacea angustifolia extract, ilex paraguariensis leaf extract, isopropyl myristate, juniperus communis fruit extract, purified water