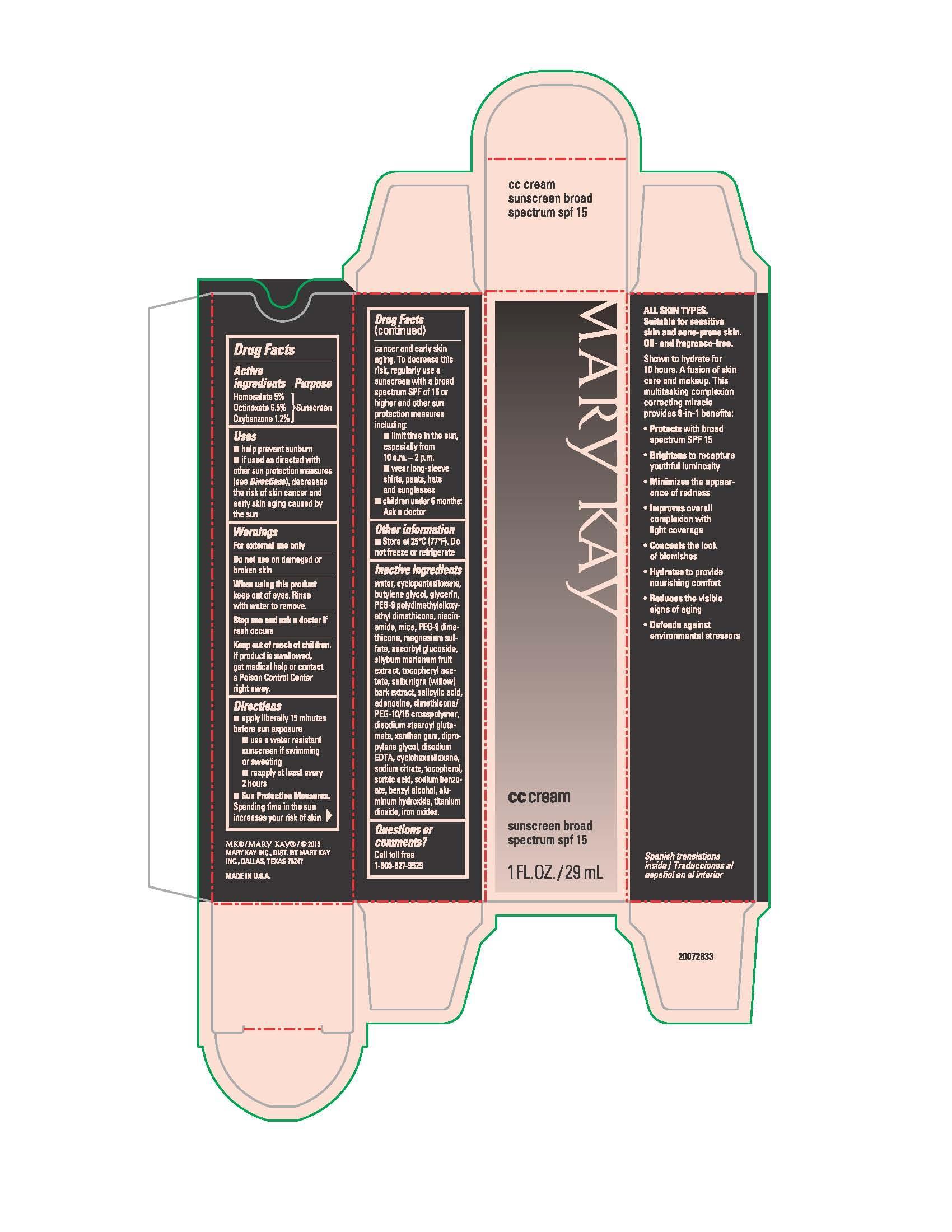
Uses
- helps prevent sunburn
- if used as directed with other sun protection measures (see Directions), decreases the risk of skin cancer and early skin aging caused by the sun
Directions
- apply liberally 15 minutes before sun exposure
- use a water resistant sunscreen if swimming or sweating
- reapply at least every 2 hours
- Sun Protection Measures. Spending time in the sun increases your risk of skin cancer and early skin aging. To decrease this risk, regularly use a sunscreen with a broad spectrum SPF of 15 or higher and other sun protection measures including:
- limit time in the sun, especially from 10 a.m. - 2 p.m.
- wear long-sleeve shirts, pants, hats and sunglasses
- children under 6 months: Ask a doctor
Inactive ingredients
water, cyclopentasiloxane, butylene glycol, glycerin, PEG-9 polydimethylsiloxyethyl dimethicone, niacinamide, mica, PEG-9 dimethicone, magnesium sulfate, ascorbyl glucoside, silybum marianum fruit extract, tocopheryl acetate, salix nigra (willow) bark extract, salicylic acid, adenosine, dimethicone/PEG-10/15 crosspolymer, disodium stearoyl glutamate, xanthan gum, dipropylene glycol, disodium EDTA, cyclohexasiloxane, sodium citrate, tocopherol, sorbic acid, sodium benzoate, benzyl alcohol, aluminum hydroxide, titanium dioxide, iron oxides