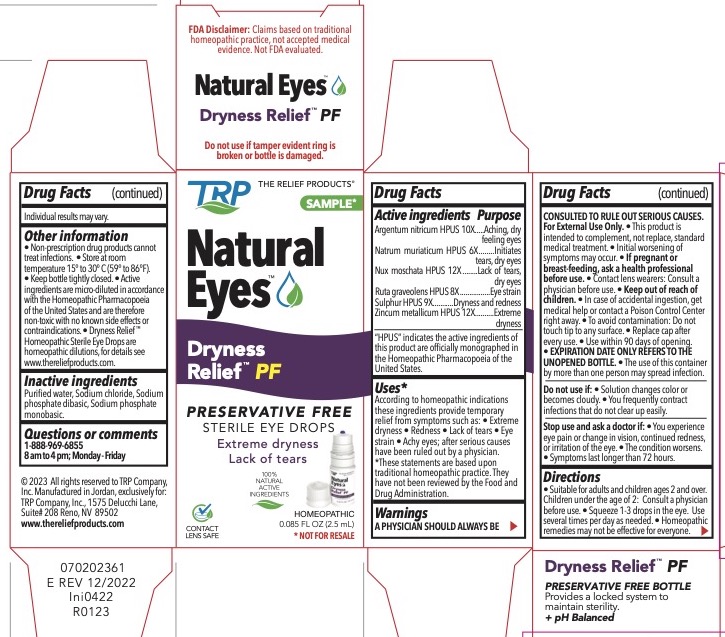
Active Ingredients
| Active Ingredients | Purpose |
| Argentum nitricum HPUS 10X | Aching, dry feeling eyes |
| Natrum muriaticum HPUS 6X | Initiates tears, dry eyes |
| Nux moschata HPUS 12X | Lack of tears, dry eyes |
| Ruta graveolens HPUS 8X | Eye strain |
| Sulphur HPUS 9X | Dryness and redness |
| Zincum metallicum HPUS 12x | Extreme dryness |
“HPUS” indicates the active ingredients are in the Homeopathic Pharmacopoeia of the United States.
Purpose
Argentum nitricum - Aching, dry feeling eyes
Natrum muriaticum - Initiates tears, dry eyes
Nux moschata - Lack of tears, dry eyes
Ruta graveolens - Eye strain
Sulphur - Dryness and redness
Zincum metallicum - Extreme dryness
Uses*
Uses:*
According to homeopathic indications these ingredients provide temporary relief from symptoms such as: • Extreme dryness • Redness • Lack of tears • Eye strain • Achy eyes; after serious causes have been ruled out by a physician. *These statements are based upon traditional homeopathic practice. They have not been reviewed by the Food and Drug Administration.
Warnings
Warnings:
A PHYSICIAN SHOULD ALWAYS BE CONSULTED TO RULE OUT SERIOUS CAUSES.
For External Use Only.
• This product is intended to complement, not replace, standard medical treatment. • Initial worsening of symptoms may occur.
Contact lens wearers consult physician prior to using.
To avoid contamination - do not touch tip to any surface.
Replace cap after every use.
Use within 30 days of opening.
EXPIRATION DATE ONLY REFERS TO THE UNOPENED BOTTLE.
The use of this container by more than one person may spread infection.
Keep out of reach of children
• Keep out of reach of children. • In case of accidental ingestion, get medical help or contact a Poison Control Center right away.
Do not use:
- If solution changes color or becomes cloudy.
- If you frequently contract infections that do not clear up easily.
Stop use and ask a doctor if:
- • You experience eye pain or change in vision, continued redness or irritation of the eye.
- • The condition worsens.
- • Symptoms last longer than 72 hours.
Directions
Directions:
• Suitable for adults and children ages 2 and over. Children under the age of 2: Consult a physician before use. • Squeeze 1-3 drops in the eye. Use several times per day as needed. • Homeopathic remedies may not be effective for everyone. Individual results may vary.
Other information:
• Non-prescription drug products cannot treat infections.
• Store at room temperature 15° to 30° C (59° to 86°F)
• Keep bottle tightly closed.
• Active ingredients are micro-diluted in accordance with the Homeopathic Pharmacopoeia of the United States and are therefore non-toxic with no known side effects or contraindications.
• Dryness Relief PF ® Homeopathic Sterile Eye Drops are homeopathic dilutions, for details see www.thereliefproducts.com