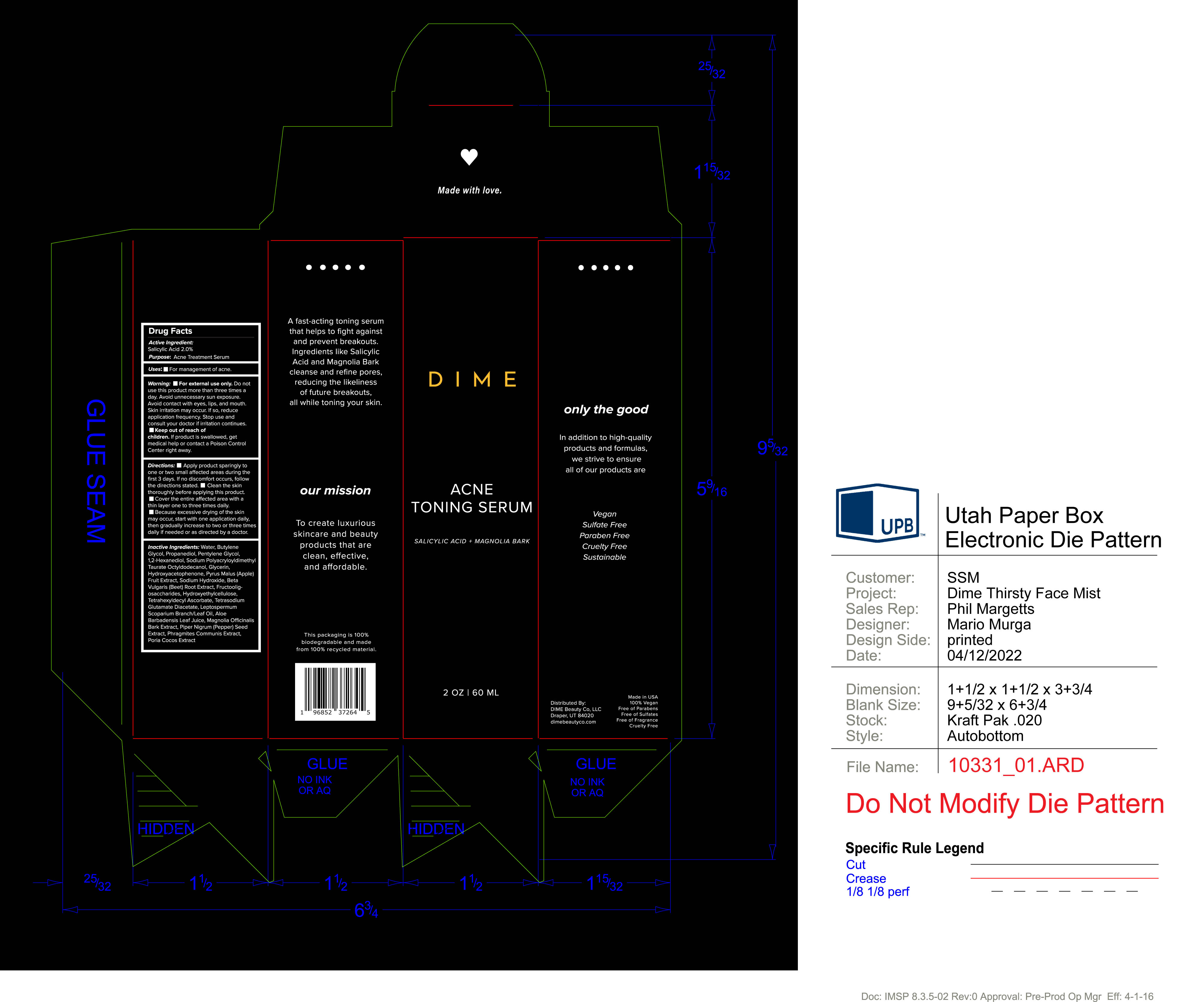
Keep out of reach of children. If product is swallowed, get medical help or contact a Poison Control Center right away.
Warning:
- For external use only. Do not use this product more than three times a day. Avoid unnecesary sun exposure. Avoid contact with eyes, lip and mouth. Skin irritation may occur. If so, reduce application frequency.
Directions:
- Apply product sparingly to one or two small affected areas during the first 3 days. If no discomfort occurs, follow the directions stated.
- Clean the skin thoroughly before applying this product.
- Cover the entire affected area with a thin layer one to three times daily..
- Because excessive drying of the skin may occur, start with one application daily, then gradually increase to two or three times daily if needed or as directed.
Inactive Ingredients: Water, Butylene Glycol, Propanediol, Pentylene Glycol, 1,2-Hexanediol, Sodium Polyacryloyldimethyl Taurate Octyldodecanol, Glycerin, Hydroxyacetophenone, Pyrus Malus (Apple) Fruit Extract, Sodium Hydroxide, Beta Vulgaris (Beet) Root Extract, Fructooligosaccharides, Hydroxyethylcellulose, Tetrahexyldecyl Ascorbate, Tetrasodium Glutamate Diacetate, Leptospermum Scoparium Branch/Leaf Oil, Aloe Barbadensis Leaf Juice, Magnolia Officinalis Bark Extract, Piper Nigrum (Pepper) Seed Extract, Phragmites Communis Extract, Poria Cocos Extract