FULL PRESCRIBING INFORMATION
1 INDICATIONS AND USAGE
1.1 Bile Acid Synthesis Disorders due to Single Enzyme Defects
CHOLBAM is indicated for the treatment of bile acid synthesis disorders due to single enzyme defects (SEDs)
1.2 Peroxisomal Disorders Including Zellweger Spectrum Disorders
CHOLBAM is indicated for adjunctive treatment of peroxisomal disorders (PDs) including Zellweger spectrum disorders in patients who exhibit manifestations of liver disease, steatorrhea or complications from decreased fat-soluble vitamin absorption.
2 DOSAGE AND ADMINISTRATION
2.1 Dosage Regimen for Bile Acid Synthesis Disorders Due to SEDs and PDs Including Zellweger Spectrum Disorders
The recommended dosage of CHOLBAM is 10 to 15 mg/kg administered orally once daily, or in two divided doses, in pediatric patients and in adults.
Tables 1 and 2 show the number of capsules that should be administered daily to approximate a dosage of 10 mg/kg/day and 15 mg/kg/day, respectively, using the available 50 mg and 250 mg capsules alone or in combination.
| 10 mg/kg/day Dosage | ||
|---|---|---|
| Body Weight (kg) | Number of 50 mg capsules | Number of 250 mg capsules |
| 4 to 6 | 1 | 0 |
| 7 to 10 | 2 | 0 |
| 11 to 15 | 3 | 0 |
| 16 to 20 | 4 | 0 |
| 21 to 25 | 0 | 1 |
| 26 to 30 | 1 | 1 |
| 31 to 35 | 2 | 1 |
| 36 to 40 | 3 | 1 |
| 41 to 45 | 4 | 1 |
| 46 to 50 | 0 | 2 |
| 51 to 55 | 1 | 2 |
| 56 to 60 | 2 | 2 |
| 61 to 65 | 3 | 2 |
| 66 to 70 | 4 | 2 |
| 71 to 75 | 0 | 3 |
| 76 to 80 | 1 | 3 |
| 15 mg/kg/day Dosage | ||
|---|---|---|
| Body Weight (kg) | Number of 50 mg capsules | Number of 250 mg capsules |
| 4 to 5 | 1 | 0 |
| 6 to 9 | 2 | 0 |
| 10 to 13 | 3 | 0 |
| 14 to 16 | 4 | 0 |
| 17 to 19 | 0 | 1 |
| 20 to 23 | 1 | 1 |
| 24 to 26 | 2 | 1 |
| 27 to 29 | 3 | 1 |
| 30 to 33 | 4 | 1 |
| 34 to 36 | 0 | 2 |
| 37 to 39 | 1 | 2 |
| 40 to 43 | 2 | 2 |
| 44 to 46 | 3 | 2 |
| 47 to 49 | 4 | 2 |
| 50 to 53 | 0 | 3 |
| 54 to 56 | 1 | 3 |
| 57 to 59 | 2 | 3 |
| 60 to 63 | 3 | 3 |
| 64 to 66 | 4 | 3 |
| 67 to 69 | 0 | 4 |
| 70 to 73 | 1 | 4 |
| 74 to 76 | 2 | 4 |
| 77 to 79 | 3 | 4 |
| 80 | 4 | 4 |
Patients with newly diagnosed, or a family history of, familial hypertriglyceridemia may have poor absorption of CHOLBAM from the intestine and require a 10% increase in the recommended dosage to account for losses due to malabsorption. The recommended dosage of CHOLBAM in patients with concomitant familial hypertriglyceridemia is 11 to 17 mg/kg orally once daily, or in two divided doses. Adequacy of the dosage regimen can be determined by monitoring the patient’s clinical response including steatorrhea and laboratory values of serum transaminases, bilirubin, and prothrombin time/international normalized ratio (PT/INR).
2.2 Treatment Monitoring
Treatment with CHOLBAM should be initiated and monitored by an experienced hepatologist or pediatric gastroenterologist.
Monitor serum aspartate aminotransferase (AST), serum alanine aminotransferase (ALT), serum gamma glutamyltransferase (GGT), alkaline phosphatase, bilirubin, and INR every month for the first 3 months, every 3 months for the next 9 months, every 6 months during the subsequent three years, and annually thereafter. Monitor more frequently during periods of rapid growth, concomitant disease, and pregnancy. Administer the lowest dose of CHOLBAM that effectively maintains liver function [see Warnings and Precautions (5.1)].
Discontinue treatment with CHOLBAM if liver function does not improve within 3 months of the start of treatment or if complete biliary obstruction develops.
Discontinue treatment with CHOLBAM at any time if there are persistent clinical or laboratory indicators of worsening liver function or cholestasis [see Warnings and Precautions (5.1)].
Concurrent elevations of serum GGT and serum ALT may indicate CHOLBAM overdose [see Overdosage (10)]. Continue to monitor laboratory parameters of liver function and consider restarting at a lower dose when the parameters return to baseline.
Assessment of serum or urinary bile acid levels using mass spectrometry is used in the diagnosis of bile acid synthesis disorders due to SEDs and PDs including Zellweger spectrum disorders. The utility of bile acid measurements in monitoring the clinical course of patients and in decisions regarding dose adjustment has not been demonstrated.
2.3 Administration Instructions
- Take CHOLBAM with food.
- Take CHOLBAM at least 1 hour before or 4 to 6 hours (or at as great an interval as possible) after a bile acid binding resin or aluminum-based antacid.
- Do not crush or chew the capsules.
- For patients unable to swallow the capsules, open the capsules and mix the contents with infant formula or expressed breast milk (for younger children), or soft food such as mashed potatoes or apple puree (for older children and adults) in order to mask any unpleasant taste:
- Hold the capsule over the prepared liquid/food, gently twist open, and allow the contents to fall into the liquid/food.
- Mix the entire capsule contents with one or two tablespoons (15 mL to 30 mL) of infant formula, expressed breast milk, or soft food such as mashed potatoes or apple puree.
- Stir for 30 seconds.
- The capsule contents will remain as fine granules in the milk or food and will not dissolve.
- Administer the mixture immediately
3 DOSAGE FORMS AND STRENGTHS
CHOLBAM is available in two capsule strengths:
- 50 mg capsule: Size number 2 Swedish orange capsule with cap imprinted with "50mg" and body imprinted with "ASK001". The capsules contain a white to off-white powder.
- 250 mg capsule: Size number 0 white capsule with a cap imprinted with "250mg" and body imprinted with "ASK002". The capsules contain a white to off-white powder.
5 WARNINGS AND PRECAUTIONS
5.1 Exacerbation of Liver Impairment
In clinical trials, evidence of liver impairment was present before treatment with CHOLBAM in approximately 86% (44/51) of patients with bile acid synthesis disorders due to SEDs and in approximately 50% (14/28) of patients with PDs including Zellweger spectrum disorders. Five of the patients (3SED and 2 PD) with liver impairment at baseline experienced worsening serum transaminases, elevated bilirubin values, or worsening cholestasis on liver biopsy following treatment. Five additional patients (2 SED and 3 PD) who did not have baseline cholestasis experienced exacerbation of their liver disease while on treatment. In patients with cirrhosis, cases of severe hepatotoxicity have also been observed following postmarket use of Cholbam Exacerbation of liver impairment by CHOLBAM in these patients cannot be ruled out.
Six patients with SEDs underwent liver transplant, including four patients diagnosed with AKR1D1 deficiency, one with 3β-HSD deficiency, and one with CYP7A1 deficiency.
Concurrent elevations of serum GGT and ALT may indicate CHOLBAM overdose [see Dosage and Administration (2.2) and Overdosage (10)].
Monitor liver function and discontinue CHOLBAM in patients who develop worsening of liver function while on treatment. Discontinue treatment with CHOLBAM at any time if there are clinical or laboratory indicators of worsening liver function or cholestasis [see Dosage and Administration (2.2)].
6 ADVERSE REACTIONS
The following clinically significant adverse reaction is described elsewhere in the labeling:
- Exacerbation of Liver Impairment [see Warnings and Precautions (5.1)]
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
Clinical safety experience with CHOLBAM consists of:
- Trial 1: a non-randomized, open-label, single-arm trial of 50 patients with bile acid synthesis disorders due to SEDs and 29 patients with PDs including Zellweger spectrum disorders. Safety data are available over the 18 years of the trial.
- Trial 2: an extension trial of 12 new patients (10 SED and 2 PD) along with 31 (21 SED and 10 PD) patients who rolled over from Trial 1. Safety data are available for 3 years and 11 months of treatment.
Adverse events were not collected systematically in either of these trials. Most patients received an oral dose of 10 to 15 mg/kg/day of CHOLBAM.
In Trial 1, among the 50 patients with SEDs, 5 patients aged 1 year or less died, which included three patients originally diagnosed with AKR1D1 deficiency, one with 3β-HSD deficiency and one with CYP7A1 deficiency. The cause of death was attributed to progression of underlying liver disease in every patient.
Of the 29 patients in Trial 1 with PDs including Zellweger spectrum disorders, 12 patients between the ages of 7 months and 2.5 years died. In the majority of these patients (8/12), the cause of death was attributed to progression of underlying liver disease or to a worsening of their primary illness.
Two additional patients in Trial 1 (1 SED and 1 PD) died who had been off study medication for more than one year with the cause of death most likely being a progression of their underlying liver disease. Of the patients who died with disease progression, laboratory testing showed abnormal serum transaminases, bilirubin, or cholestasis on liver biopsy suggesting worsening of their underlying cholestasis.
In Trial 2, among the 31 patients with SED, two patients (1 new patient and 1 who rolled over from Trial 1) died. The cause of death in both cases was unrelated to their primary treatment or progression of their underlying liver disease.
Of the 12 patients with PD in Trial 2, four patients died between the ages of 4 and 8 years (1 new patient and 3 who rolled over from Trial 1). The cause of death in three of these patients was attributed to progression of underlying liver disease or to a worsening of their primary illness.
Seven patients in Trial 1(4 SED and 3 PD) and 3 patients in Trial 2 (1 SED and 2 PD) experienced worsening serum transaminases, elevated bilirubin values, or worsening cholestasis on liver biopsy during treatment [see Warnings and Precautions (5.1)].
There were 12 adverse reactions reported across 9 patients in the trials, with diarrhea being the most common reaction in approximately 2% of the patient population. All other adverse reactions represented 1% of the patient population. The breakdown by trial follows:
| Adverse Reactions | Trial 1 | Trial 2 * | Overall n (%) |
|---|---|---|---|
|
|||
| Diarrhea | 1 | 2 * | 3 (2) |
| Reflux Esophagitis | 1 | 0 | 1 (1) |
| Malaise | 1 | 0 | 1 (1) |
| Jaundice | 1 | 0 | 1 (1) |
| Skin lesion | 1 | 0 | 1 (1) |
| Nausea | 0 | 1 * | 1 (1) |
| Abdominal Pain | 0 | 1 * | 1 (1) |
| Intestinal Polyp | 0 | 1 * | 1 (1) |
| Urinary Tract Infection | 0 | 1 * | 1 (1) |
| Peripheral Neuropathy | 0 | 1 | 1 (1) |
Only one of the reactions (peripheral neuropathy) resulted in discontinuation of medication for a patient in Trial 2. An additional five SED patients (3 from Trial 1 and 2 from Trial 2) and 1 PD patient (Trial 1) discontinued medication and withdrew from the study due to a worsening of their primary disease.
The development of symptomatic cholelithiasis requiring cholecystectomy has been reported in a single patient with 3β-HSD deficiency.
6.2 Postmarketing Experience
The following adverse reactions have been identified during post-approval use of CHOLBAM. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their relative frequency or to establish a causal relationship to CHOLBAM exposure.
- Gastrointestinal disorders: discomfort and distention, emesis, constipation
- General disorders and administrative site conditions: pyrexia/fever
- Skin and subcutaneous tissue disorders: rash
7 DRUG INTERACTIONS
7.1 Effects of other drugs on CHOLBAM
Drug interactions with CHOLBAM mainly relate to agents capable of interrupting the enterohepatic circulation of bile acids.
Inhibitors of Bile Acid Transporters
Avoid concomitant use of inhibitors of the bile salt efflux pump (BSEP) such as cyclosporine. Concomitant medications that inhibit canalicular membrane bile acid transporters such as the BSEP may exacerbate accumulation of conjugated bile salts in the liver and result in clinical symptoms. If concomitant use is deemed necessary, monitoring of serum transaminases and bilirubin is recommended.
Bile acid binding resins such as cholestyramine, colestipol, or colesevelam adsorb and reduce bile acid absorption and may reduce the efficacy of CHOLBAM. Take CHOLBAM at least 1 hour before or 4 to 6 hours (or at as great an interval as possible) after a bile acid binding resin [see Dosage and Administration (2.3)].
Aluminum-based antacids have been shown to adsorb bile acids in vitro and can reduce the bioavailability of CHOLBAM. Take CHOLBAM at least 1 hour before or 4 to 6 hours (or at as great an interval as possible) after an aluminum-based antacid [see Dosage and Administration (2.3)].
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
There is a pregnancy surveillance program that monitors pregnancy outcomes in women exposed to CHOLBAM during pregnancy [COCOA Registry (ChOlbam: Child and mOther's heAlth)]. Women who become pregnant during CHOLBAM treatment are encouraged to enroll. Patients or their health care provider should call 1-844-20C-OCOA or 1-844-202-6262 to enroll.
No studies in pregnant women or animal reproduction studies have been conducted with CHOLBAM.
Limited published case reports discuss pregnancies in women taking cholic acid for 3β-HSD deficiency resulting in healthy infants. These reports may not adequately inform the presence or absence of drug-associated risk with the use of CHOLBAM during pregnancy. The background risk of major birth defects and miscarriage for the indicated population is unknown. However, the background risk in the U.S. general population of major birth defects is 2-4% and of miscarriage is 15-20% of clinically recognized pregnancies.
8.2 Lactation
Endogenous cholic acid is present in human milk. Clinical lactation studies have not been conducted to assess the presence of CHOLBAM in human milk, the effects of CHOLBAM on the breastfed infant, or the effects of CHOLBAM on milk production. There are no animal lactation data and no data from case reports available in the published literature. The developmental and health benefits of breastfeeding should be considered along with the mother's clinical need for CHOLBAM and any potential adverse effects on the breastfed infant from CHOLBAM or from the underlying maternal condition.
8.4 Pediatric Use
The safety and effectiveness of CHOLBAM have been established in pediatric patients 3 weeks of age and older for the treatment of bile acid synthesis disorders due to SEDs and for adjunctive treatment of patients with PDs including Zellweger spectrum disorders who exhibit manifestations of liver disease, steatorrhea, or complications from decreased fat-soluble vitamin absorption [see Clinical Studies (14)].
8.5 Geriatric Use
Clinical studies of CHOLBAM did not include any patients aged 65 years and over. It is not known if elderly patients respond differently from younger patients.
8.6 Hepatic Impairment
Discontinue treatment with CHOLBAM if liver function does not improve within 3 months of the start of treatment.
Discontinue treatment with CHOLBAM at any time if there are clinical or laboratory indicators of worsening liver function or cholestasis [see Warnings and Precautions (5.1), Overdosage (10), and Nonclinical Toxicology (13.2)]. Continue to monitor laboratory parameters of liver function and consider restarting at a lower dose when the parameters return to baseline.
10 OVERDOSAGE
Concurrent elevations of serum GGT and ALT may indicate CHOLBAM overdose. If an overdose is suspected, discontinue CHOLBAM and treat symptoms. Continue to monitor laboratory parameters of liver function and consider restarting at a lower dose when the parameters return to baseline [see Dosage and Administration (2.2) and Warnings and Precautions (5.1)].
11 DESCRIPTION
Cholic acid is a bile acid. The chemical formula is C24H40O5, the molecular weight is 408.57 and the chemical structure is:
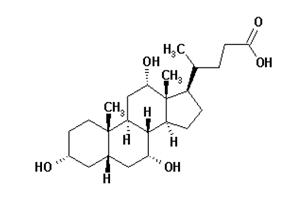
Cholic acid is a white to off-white powder. It is practically insoluble in water and in 0.1 M HCl at 20°C and is sparingly soluble in 0.1 M NaOH at 20°C. It is soluble in glacial acetic acid, alcohols, and acetone. A saturated solution in water at 20°C has a pH of 4.4.
CHOLBAM capsules contain 50 mg or 250 mg of cholic acid as the active ingredient in size 2 Swedish orange or size 0 white opaque gelatin capsules, respectively. Inactive ingredients in CHOLBAM include crospovidone, magnesium stearate, and silicified microcrystalline cellulose.
The size 2 capsule shells contain gelatin, red iron oxide and titanium dioxide, and the size 0 capsule shells contain gelatin and titanium dioxide. CHOLBAM is administered orally.
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
Cholic acid is a primary bile acid synthesized from cholesterol in the liver. In bile acid synthesis disorders due to SEDs in the biosynthetic pathway, and in PDs including Zellweger spectrum disorders, deficiency of primary bile acids leads to unregulated accumulation of intermediate bile acids and cholestasis. Bile acids facilitate fat digestion and absorption by forming mixed micelles and facilitate absorption of fat-soluble vitamins in the intestine.
Endogenous bile acids including cholic acid enhance bile flow and provide the physiologic feedback inhibition of bile acid synthesis. The mechanism of action of cholic acid has not been fully established; however, it is known that cholic acid and its conjugates are endogenous ligands of the nuclear receptor, farnesoid X receptor (FXR). FXR regulates enzymes and transporters that are involved in bile acid synthesis and in the enterohepatic circulation to maintain bile acid homeostasis under normal physiologic conditions.
12.3 Pharmacokinetics
Orally administered cholic acid is subject to the same metabolic pathway as endogenous cholic acid.
Cholic acid is absorbed by passive diffusion along the length of the gastrointestinal tract. Once absorbed, cholic acid enters into the body's bile acid pool and undergoes enterohepatic circulation mainly in conjugated forms.
In the liver, cholic acid is conjugated with glycine or taurine by bile acid-CoA synthetase and bile acid-CoA:amino acid N-acetyltransferase. Conjugated cholic acid is actively secreted into bile by the BSEP, and then released into the small intestines, along with other components of bile.
Conjugated cholic acid is mostly re-absorbed in the ileum mainly by the apical-sodium-dependent-bile acid transporter, passed back to the liver by transporters including sodium-taurocholate cotransporting polypeptide and organic anion transport protein and enters another cycle of enterohepatic circulation. Any conjugated cholic acid not absorbed in the ileum passes into the colon where deconjugation and 7-dehydroxylation are mediated by bacteria to form cholic acid and deoxycholic acid, which may be re-absorbed in the colon or excreted in the feces. The loss of cholic acid is compensated by de-novo synthesis of cholic acids from cholesterol to maintain the bile acid pool in healthy subjects.
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
Carcinogenicity, genetic toxicology, and nonclinical fertility studies have not been performed with cholic acid.
13.2 Animal Toxicology and/or Pharmacology
In the PEX2 -/- mouse model of peroxisomal disorders, feeding with a combination of cholic acid and ursodeoxycholic acid normalized C24 bile acid concentrations in bile to that of untreated control animals. Although growth was only mildly improved, there was near complete normalization of stool fat content, resolution of steatorrhea, and improved survival. Bile acid feeding reduced the number of cholestatic deposits in bile ducts and alleviated cholangitis but exacerbated the degree of hepatic steatosis and mitochondrial and cellular damage in the peroxisome-deficient livers of these animals.
14 CLINICAL STUDIES
14.1 Bile Acid Synthesis Disorders due to Single Enzyme Defects
The effectiveness of CHOLBAM at dosages of 10 to 15 mg/kg per day in patients with SEDs was assessed in:
- Trial 1: a non-randomized, open-label, single-arm trial in 50 patients over an 18-year period.
- Trial 2: an extension trial of 12 new patients along with 21 patients who rolled-over from Trial 1 (n=33 total). Efficacy data are available for 21 months of treatment.
- A published case series of 15 patients patients with SEDs and 3 patients with PDs.
Enrollment criteria in Trials 1 and 2 were based on abnormal urinary bile acid by Fast Atom Bombardment ionization - Mass Spectrometry (FAB-MS).
Pre- and post-treatment liver biopsies were performed in a limited number of patients. Documentation of adherence to treatment, concomitant medications, and response to treatment were incomplete during Trial 1. Additional interventions in some patients included supplementation with fat-soluble vitamins, as dictated by the patient's clinical signs and symptoms.
On average, patients were 4 years of age at the start of cholic acid treatment (range three weeks to 36 years). The majority of patients were treated for an average of 310 weeks (6 years). Patient ages at the end of treatment ranged from 19 to 36 years.
These trials were carried out over many years, and data are not available on all patients. Thirty-nine patients in Trial 1 and 5 new patients in Trial 2 received at least one dose of CHOLBAM and had sufficient data available to assess baseline liver function and effects of CHOLBAM treatment. A responder analysis was performed to determine the response to treatment with CHOLBAM.
Response to CHOLBAM treatment was assessed by the following laboratory criteria:
- ALT or AST values reduced to less than 50 U/L, or baseline levels reduced by 80%;
- total bilirubin values reduced to less than or equal to 1 mg/dL; and
- no evidence of cholestasis on liver biopsy;
and the following clinical criteria:
- body weight increased by 10% or stable at greater than the 50th percentile; and
- survival for greater than 3 years on treatment or alive at the end of Trial 2
CHOLBAM responders were defined as patients who either:
- met at least two laboratory criteria and were alive at the last follow-up; or
- met at least one laboratory criterion, had increased body weight and were alive at the last follow-up.
Overall, 28 of 44 patients (64%) were responders. The breakdown by defect type is as follows:
| Single Enzyme Defect | Responders/Number Treated (%) |
|---|---|
|
|
| 3β-HSD | 22/37 (59%) |
| AKR1D1 | 3/4 (75%) |
| CTX | 2/2 (100%) |
| AMACR | 1/1 (100%) |
| CYP7A1 | N/A* |
| Smith-Lemli-Opitz | N/A* |
Among SED responsive patients, 45% of the responders met the two clinical criteria plus 1 to 3 laboratory criteria and 55% met the weight criteria.
Only six patients had pre- and post-treatment liver biopsies in Trial 1. Where biopsies were available, pre-treatment biopsies showed varying degrees of inflammation, bridging fibrosis, and giant cell formation. Post-treatment biopsies generally showed reduced or absent inflammation and reduced or absent giant cell formation. Fibrosis remained but did not progress.
It is difficult to evaluate long term survival in patients with SEDs since there is little natural history survival data for comparison. Overall, 41 of 62 (67%) patients with SEDs survived greater than 3 years from trial entry. Thirteen of these 41 patients (32%) survived for 10 to 24 years on treatment.
Four patients in Trial 1 underwent liver transplant, including two patients diagnosed with AKR1D1 deficiency, one with 3β-HSD deficiency, and one with CYP7A1 deficiency and two patients in Trial 2, both with AKR1D1.
CHOLBAM’s effects on extrahepatic manifestations of SEDs, such as neurologic symptoms have not been established.
A published report of a case series described 15 patients with SEDs; thirteen were diagnosed with 3β-HSD deficiency and two with AKR1D1 deficiency by mass spectrometry and gene sequencing. All patients were treated with cholic acid with a median duration of treatment of 12.4 years (range 5.6 to 15 years). Therapy started at a median age of 3.9 years (range 0.3 to 13.1 years). The mean dose at the start of cholic acid treatment was 13 mg/kg and the mean dose at last follow up was 6 mg/kg. Eight patients were initially treated with oral ursodeoxycholic acid prior to receiving a diagnosis of bile acid synthesis defect, after which they were switched to cholic acid. Initial signs and symptoms included jaundice, hepatosplenomegaly, steatorrhea, or symptoms related to deficiency of a fat-soluble vitamin (K, D or E).
Of the 8 patients who received ursodeoxycholic acid initially, the six with 3β-HSD deficiency demonstrated mild clinical improvement. Following treatment with cholic acid, all patients experienced resolution of their pre-existing jaundice and steatorrhea, and all but one experienced resolution of hepatosplenomegaly. Weight and height improved, and sexual maturation progressed normally in all patients. Liver biopsies were performed in 14 patients after at least 5 years of cholic acid treatment and all showed resolution of cholestasis. In one patient with 3β-HSD deficiency, biliary bile acid analysis while on cholic acid therapy showed enrichment of the bile with cholic acid.
14.2 Peroxisomal Disorders including Zellweger Spectrum Disorders
The effectiveness of CHOLBAM at a dosage of 10 to 15 mg/kg per day in patients with PDs including Zellweger spectrum disorders was assessed in patients in the same trials described in section 14.1.
- Trial 1 treated 29 patients with PDs over an 18‑year period.
- Trial 2 treated 2 new patients along with 10 patients who rolled over from Trial 1 (n=12 total). Efficacy data are available from Trial 2 for 21 months of treatment.
- Additional efficacy data were obtained from published case reports of 3 patients.
Enrollment criteria in Trials 1 and 2 were based on abnormal urinary bile acids analysis by FAB-MS and a neurologic exam. Most patients received concomitant DHA (docosahexaenoic acid) and Vitamins A, D, E, and K. Documentation of adherence to treatment, concomitant medications, and response to treatment were incomplete during Trial 1.
The majority of patients (80%, 25/31) were less than 2 years of age at the start of CHOLBAM treatment (range 3 weeks to 10 years). The majority of patients were treated for an average of 254 weeks (4.8 years).
Sufficient data were available to assess baseline liver function and effects of CHOLBAM treatment in 23 patients in Trial 1 and in one new patient in Trial 2. A responder analysis was performed in the patients who had received at least one dose of CHOLBAM and had sufficient data available to assess baseline liver impairment.
Response to CHOLBAM treatment was assessed by the following laboratory criteria:
- ALT or AST values reduced to less than 50 U/L, or baseline levels reduced by 80%;
- total bilirubin values reduced to less than or equal to 1 mg/dL; and
- no evidence of cholestasis on liver biopsy;
and the following clinical criteria:
- body weight increased by 10% or stable at greater than the 50th percentile; and
- survival for greater than 3 years on treatment or alive at the end of Trial 2
CHOLBAM responders were defined as patients who either:
- met at least two laboratory criteria and were alive at the last follow-up; or
- met at least one laboratory criterion, had increased body weight and were alive at the last follow-up.
Overall, 11 of 24 patients (46%) were responders. The breakdown by disorder is as follows:
| Peroxisomal Disorder | Responders/Number Treated (%) |
|---|---|
| Neonatal Adrenoleukodystropyhy | 3/6 (50%) |
| Generalized Peroxisomal Disorder | 1/1 (100%) |
| Refsum Disease | 3/4 (75%) |
| Zellweger Syndrome | 3/8 (38%) |
| Peroxisomal Disorder, Type Unknown | 1/5 (20%) |
Among responsive patients with PDs, 38% of the responders met the two clinical criteria plus 1 to 3 laboratory criteria and 63% met the weight criteria. There were no PD patients that underwent liver transplant.
No evidence of improvement in survival over that seen in historical controls could be demonstrated from the data presented. Overall, 13 of 31 patients (42%) survived greater than 3 years from the time of trial entry. Eight of these 13 patients (62%) survived 10 to 17 years on treatment.
Nine patients had both pre- and post-treatment liver biopsies. One patient showed improvement in histology, while the majority of patients remained unchanged. Two patients demonstrated worsening histology, which was consistent with a worsening of other liver laboratory parameters (bilirubin, serum transaminase values).
CHOLBAM’s effects on extrahepatic manifestations of PDs including Zellweger spectrum disorders, such as neurologic symptoms have not been established.
One patient, who did not have cholestasis on pre-treatment liver biopsy, developed cholestasis on treatment with CHOLBAM and subsequently died.
In case reports from the literature, a 6-month-old patient with Zellweger syndrome treated with a combination of cholic and chenodeoxycholic acids experienced normalization of serum transaminases and bilirubin, improvement in liver histology, reduced serum and urinary atypical bile acid intermediates, and improvement in steatorrhea and growth. Two patients with Zellweger syndrome treated with oral bile acids showed decreased serum transaminases.
16 HOW SUPPLIED/STORAGE AND HANDLING
CHOLBAM capsules are available as two-piece gelatin capsules with a Swedish orange cap imprinted with "50mg" and Swedish orange body imprinted with "ASK001". The capsules contain a white or off-white powder and are supplied in bottles of:
- 90 capsules (NDC 45043-001-02)
17 PATIENT COUNSELING INFORMATION
Exacerbation of Liver Impairment [see Warnings and Precautions (5.1)]
- Advise patients that they will need to undergo laboratory testing periodically while on treatment to assess liver function.
- Advise patients that CHOLBAM may worsen liver impairment and that they should immediately report to their health care provider any symptoms associated with liver impairment (e.g., yellowing of the skin or the whites of the eye, dark or tea-colored urine, pain on the right side of stomach, bleeding or bruising occurs more easily than normal, or increased lethargy)
Administration [see Dosage and Administration (2.3)]
- to take CHOLBAM with food.
- to take CHOLBAM at least one hour before or 4 to 6 hours after taking a bile acid binding resin or an aluminum-based antacid.
- not to crush or chew the capsules.
- for infants and children who cannot swallow capsules, the capsules can be opened and the contents mixed with either infant formula or expressed breast milk (for younger children), or soft food such as mashed potatoes or apple puree (for older children and adults) in order to mask any unpleasant taste:
- Hold the capsule over the prepared liquid/food, gently twist open, and allow the contents to fall into the liquid/food.
- Mix the entire capsule contents with one or two tablespoonfuls (15 mL to 30 mL) of infant formula, expressed breast milk, or soft food such as mashed potatoes or apple puree.
- Stir for 30 seconds.
- The capsule contents will remain as fine granules in the milk or food and will not dissolve.
- Administer the mixture immediately.
Pregnancy Registry [see Use in Specific Populations (8.1)]
Inform patients about the pregnancy surveillance program that monitors pregnancy outcomes in women exposed to CHOLBAM during pregnancy.
CHOLBAM® is a registered trademark of Travere Therapeutics, Inc.
Manufactured by:
Patheon France SA
38300 Bourgoin-Jallieu, France
Manufactured for:
Manchester Pharmaceuticals, Inc. a wholly owned subsidiary of Travere Therapeutics, Inc
San Diego, CA 92130
PRINCIPAL DISPLAY PANEL - 50 mg Capsule Bottle Label
NDC 45043- 001-02
90 capsules
Cholbam™
(cholic acid) capsules
50 mg
Rx Only
Manufactured for:
Manchester Pharmaceuticals, Inc.,
San Diego, CA 92130
Manufactured by:
Patheon France SA
38300 Bourgoin-Jallieu, France
For product information please call 844-246-5226.
PRINCIPAL DISPLAY PANEL - 250 mg Capsule Bottle Label
NDC 45043- 002-02
90 capsules
Cholbam™
(cholic acid) capsules
250 mg
Rx Only
Manufactured for:
Manchester Pharmaceuticals, Inc.,
San Diego, CA 92130
Manufactured by:
Patheon France SA
38300 Bourgoin-Jallieu, France
For product information please call 844-246-5226.