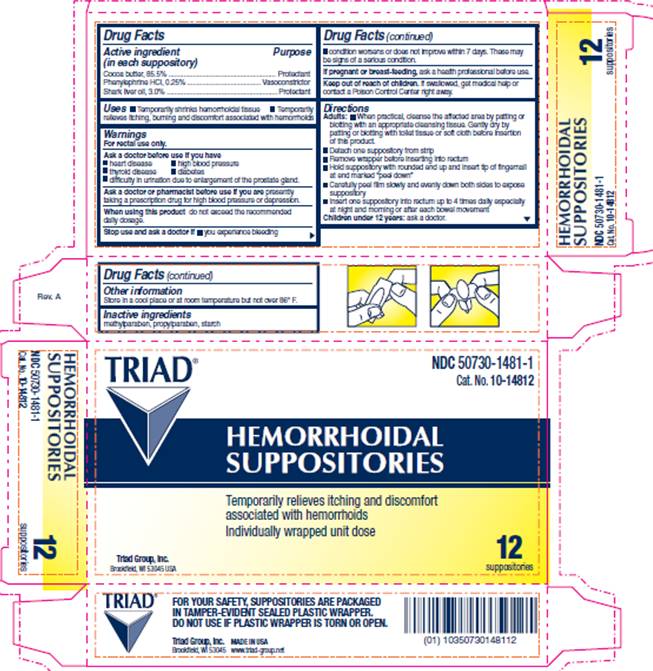
USES
- temporarily shrinks hemorrhoidal tissue
- temporarily relieves itching burning and discomfort associated with hemorrhoids
WARNINGS
For rectal use only.
Ask a doctor before use if you have
- heart disease
- thyroid disease
- difficulty in urination due to enlargement of the prostate gland
- high blood pressure
- diabetes
Ask a doctor or pharmacist before use if
you are presently taking a prescription drug for high blood pressure or depression.
DIRECTIONS
Adults:- When practical, cleanse the affected area by patting or blotting with an appropriate cleansing tissue. Gently dry by patting or blotting with toilet tissue or soft cloth before insertion of this product.
- Detach one suppository from strip
- Remove wrapper before inserting into rectum
- Hold suppository with rounded end up and insert tip of fingernail at end marked "peel down"
- Carefully peel film slowly and evenly down both sides to expose suppository
- Insert one suppository into rectum up to 4 times daily especially at night and morning or after each bowel movement
PACKAGE INFORMATION
NDC 50730-1481-1Cat. No. 10-14812
TRIAD®
HEMORRHOIDAL
SUPPOSITORIES
Temporarily relieves itching and discomfort associated with hemorrhoids
Individually wrapped unit dose
12 suppositories
Triad Group, Inc.
Brookfield, WI 53045 USA
FOR YOUR SAFETY, SUPPOSITORIES ARE PACKAGED IN TAMPER-EVIDENT SEALED PLASTIC WRAPPER.
DO NOT USE IF PLASTIC WRAPPER IS TORN OR OPEN.