ACTIVE INGREDIENT
(in each gram)
Bacitracin zinc, USP 400 units
Neomycin sulfate, USP 3.5 mg
Polymyxin B sulfate, USP 5,000 units
STOP USE AND ASK A DOCTOR IF
- condition persists or gets worse
- a rash or other allergic reaction develops
KEEP OUT OF REACH OF CHILDREN
If swallowed, get medical help or contact a Poison Control Center right away.
DIRECTIONS
- clean affected area
- apply a small amount of this product (an amount equal to the surface area of the tip of a finger) on the area 1 to 3 times daily
- may be covered with a sterile bandage
OTHER INFORMATION
- Store at controlled room temperature 59º - 86ºF (15º - 30ºC).
- Protect from freezing.
- Before using any medication, read all label directions. Keep carton, it contains important information.
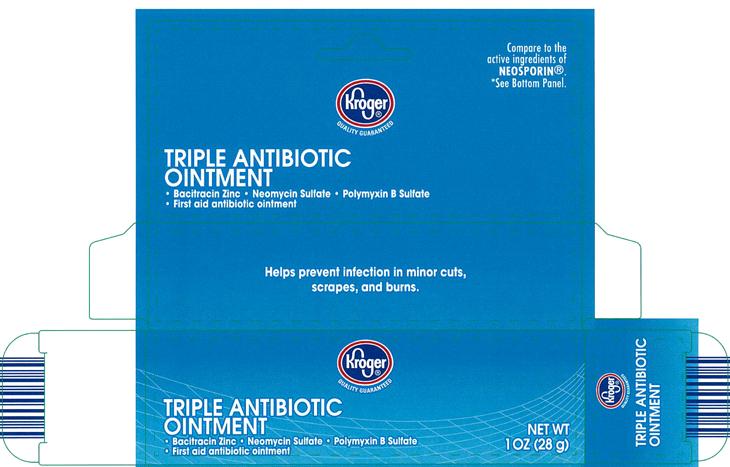
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
Kroger®
Quality Guaranteed
Compare to the active ingredients of NEOSPORIN®.*See bottom panel.
TRIPLE ANTIBIOTIC OINTMENT
• Bacitracin Zinc • Neomycin Sulfate • Polymyxin B Sulfate
• First aid antibiotic ointment
NET WT 1 OZ (28 g)
01790411B1 VC110431

CARTON LABEL