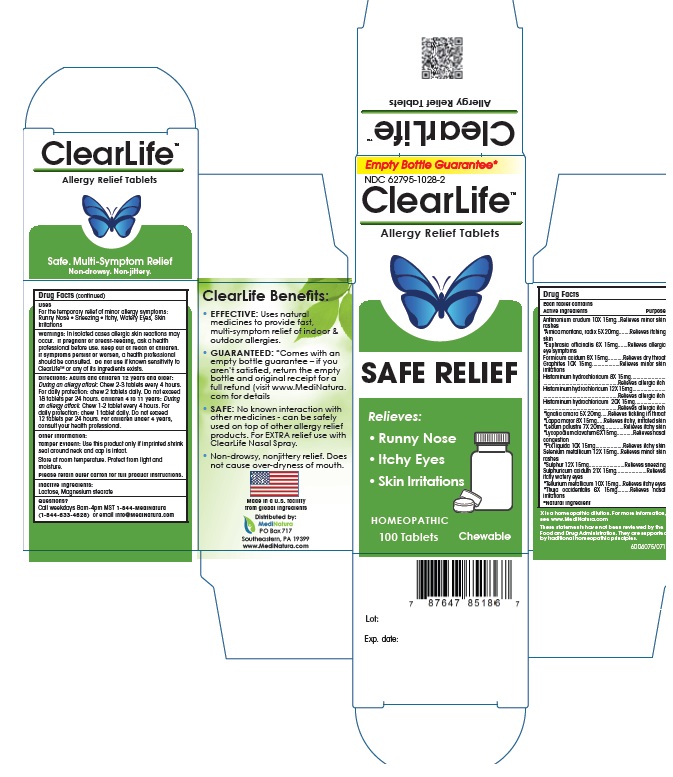
INDICATIONS AND USAGE
For the temporary relief of minor allergy symptoms:
- Runny nose
- Watery eyes
- Skin irritations
WARNINGS
If pregnant or breast-feeding, ask a healthcare provider before use. Keep out of reach of children. If symptoms persist or worsen, a healthcare provider should be consulted. Do not use if known sensitivity to ClearLife™ or any of its ingredients exists.
DOSAGE AND ADMINISTRATION
Standard Dosage:
Adults and children 12 years and older: 3 tablets per day, taking 1 tablet every 4 to 6 hours.
Children 4 to 11 years: 2 tablets per day, taking 1 tablet every 6 to 8 hours.
Children under 4 years, consult your healthcare provider.
Initial Dosage:
Adults and children 12 years and older: 1 tablet every 1/2 to 1 hour, until symptoms lessen, then continue with standard dosage. Do not exceeed 12 tablets in 24 hours.
Children 4 to 11 years: 1 tablet every 1/2 to 1 hour, until symptoms lessen, then continue with standard dosage. Do not exceed 8 tablets in 24 hours.
Children under 4 years, consult your healthcare provider.
Allow tablets to dissolve completely in the mouth, do not swallow.
ACTIVE INGREDIENTS
Active Ingredients: Each tablet contains: Antimonium crudum 10X, *Arnica montana, radix 5X,*Euphrasia officinalis 6X, Formicum acidum 8X, Graphites 10X, Histaminum hydrochloricum 8X, Histaminum hydrochloricum 12X, Histaminum hydrochloricum 20X, *Ignatia amara 5X, *Lappa major 8X, *Ledum palustre 7X, *Lycopodium clavatum 6X, *Pix liquida 10X, Selenium metallicum 12X, *Sulphur 12X, Sulphuricum acidum 21X, *Tellurium metallicum 10X, *Thuja occidentalis 6X.
*Natural Ingredients
PURPOSE
Antimonium crudum 10X..............................Relieves skin rashes
Arnica montana, radix 6X............................Relieves itching skin
Euphrasia officinalis 6X..............................Relieves allergic eye symptoms
Formicum acidum 8X...................................Relieves dry throat
Graphites 10X.............................................Relieves skin irritations
Histaminum hydrochloricum 8X, 12X, 20X....Relieves allergic itch
Ignatia amara 5X.......................................Relieves tickling in throat
Lappa major 8X..........................................Relieves itchy, Irritated skin
Ledum palustre 7X.....................................Relieves Itchy skin
Lycopodium clavatum 6X............................Relieves nasal congestion
Pix liquida 10X...........................................Relieves itchy skin
Selenium metallicum 12X...........................Relieves skin rashes
Sulphur 12X...............................................Relieves sneezing
Sulphuricum acidum 21X............................Relieves itchy, watery eyes
Tellurium metallicum 10X...........................Relieves itchy eyes
Thuja occidentalis 6X.................................Relieves nasal irritations