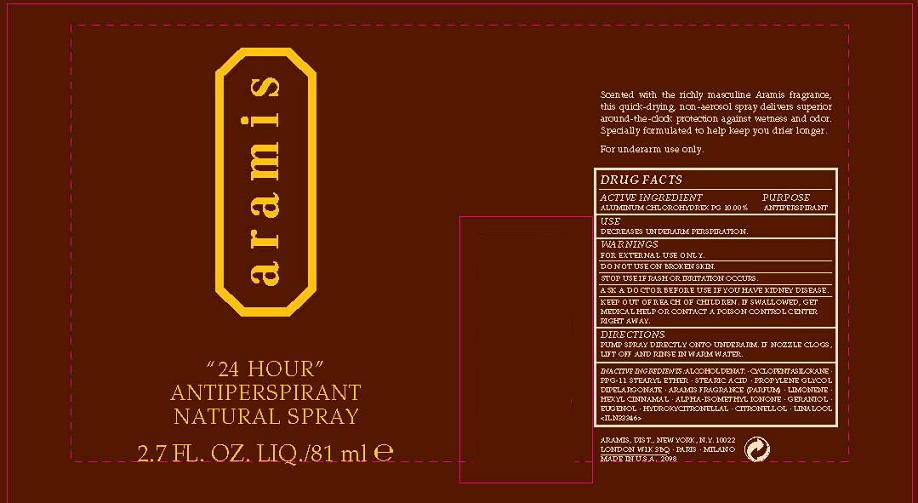
24 HOUR ANTIPERSPIRANT NATURAL - aluminum zirconium tetrachlorohydrex gly spray
ARAMIS INC.
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
ACTIVE INGREDIENT: ALUMINUM CHLOROHYDREX PG 10.00%
INACTIVE INGREDIENTS: ALCOHOL DENAT. [] CYCLOPENTASILOXANE [] PPG-11 STEARYL ETHER [] STEARIC ACID [] PROPYLENE GLYCOL DIPELARGONATE [] ARAMIS FRAGRANCE (PARFUM) [] LIMONENE [] HEXYL CINNAMAL [] ALPHA-ISOMETHYL IONONE [] GERANIOL [] EUGENOL [] HYDROXYCITRONELLAL [] CITRONELLOL [] LINALOOL
USE: REDUCES UNDERARM WETNESS
WARNINGS:
- FOR EXTERNAL USE ONLY
-
DO NOT USE ON BROKEN SKIN
-
STOP USE IF IRRITATION OCCURS
ASK A DOCTOR BEFORE USE IF YOU HAVE KIDNEY DISEASE.
KEEP OUT OF REACH OF CHILDREN. IF SWALLOWED, GET MEDICAL HELP OR CONTACT A POISON CONTROL CENTER IMMEDIATELY.
DIRECTIONS: PUMP SPRAY DIRECTLY ONTO UNDERARM. IF NOZZLE CLOGS, LIFT OFF AND RINSE IN WARM WATER.
PRINCIPAL DISPLAY PANEL:
ARAMIS
24 HOUR
ANTIPERSPIRANT
NATURAL SPRAY
NT WT 2.7 OZ/ 81 ML
ARAMIS DISTR,
NY, NY 10022
ARAMIS INC.
