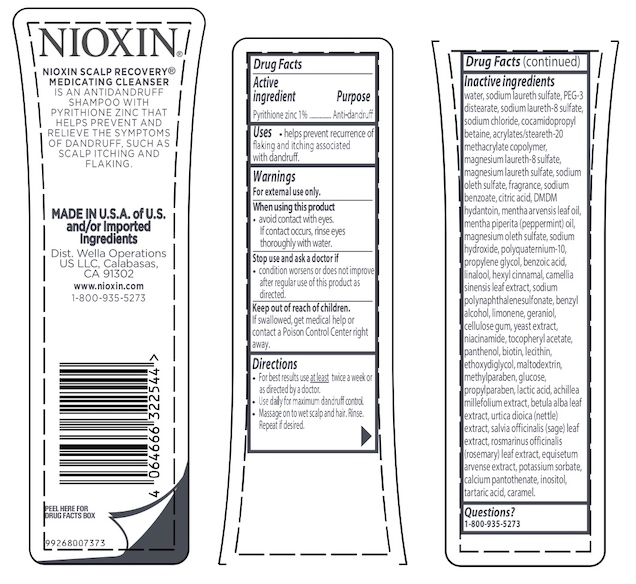
Warnings
For external use only.
When using this product
- avoid contact with eyes. If contact occurs, rinse eyes thoroughly with water.
Directions
- For best results use at least twice a week or as directed by a doctor.
- Use daily for maximum dandruff control.
- Massage on to wet scalp and hair. Rinse. Repeat if desired.
Inactive ingredients water, sodium laureth sulfate, PEG-3 distearate, sodium laureth-8 sulfate, sodium chloride, cocamidopropyl betaine, acrylates/steareth-20 methacrylate copolymer, magnesium laureth-8 sulfate, magnesium laureth sulfate, sodium oleth sulfate, fragrance, sodium benzoate, citric acid, DMDM hydantoin, mentha arvensis leaf oil, mentha piperita (peppermint) oil, magnesium oleth sulfate, sodium hydroxide, polyquaternium-10, propylene glycol, benzoic acid, linalool, hexyl cinnamal, camellia sinensis leaf extract, sodium polynaphthalenesulfonate, benzyl alcohol, limonene, geraniol, cellulose gum, yeast extract, niacinamide, tocopheryl acetate, panthenol, biotin, lecithin, ethoxydiglycol, maltodextrin, methylparaben, glucose, propylparaben, lactic acid, achillea millefolium extract, betula alba leaf extract, urtica dioica (nettle) extract, salvia officinalis (sage) leaf extract, rosmarinus officinalis (rosemary) leaf extract, equisetum arvense extract, potassium sorbate, calcium pantothenate, inositol, tartaric acid, caramel.
NIOXIN SCALP RECOVERY®
MEDICATING CLEANSER
IS AN ANTIDANDRUFF SHAMPOO WITH PYRITHIONE ZINC THAT HELPS PREVENT AND RELIEVE THE SYMPTOMS OF DANDRUFF, SUCH AS SCALP ITCHING AND FLAKING.
MADE IN U.S.A. of U.S. and/or imported ingredients
Dist. Wella Operations US LLC, Calabasas, CA 91302
www.nioxin.com
1-800-935-5273