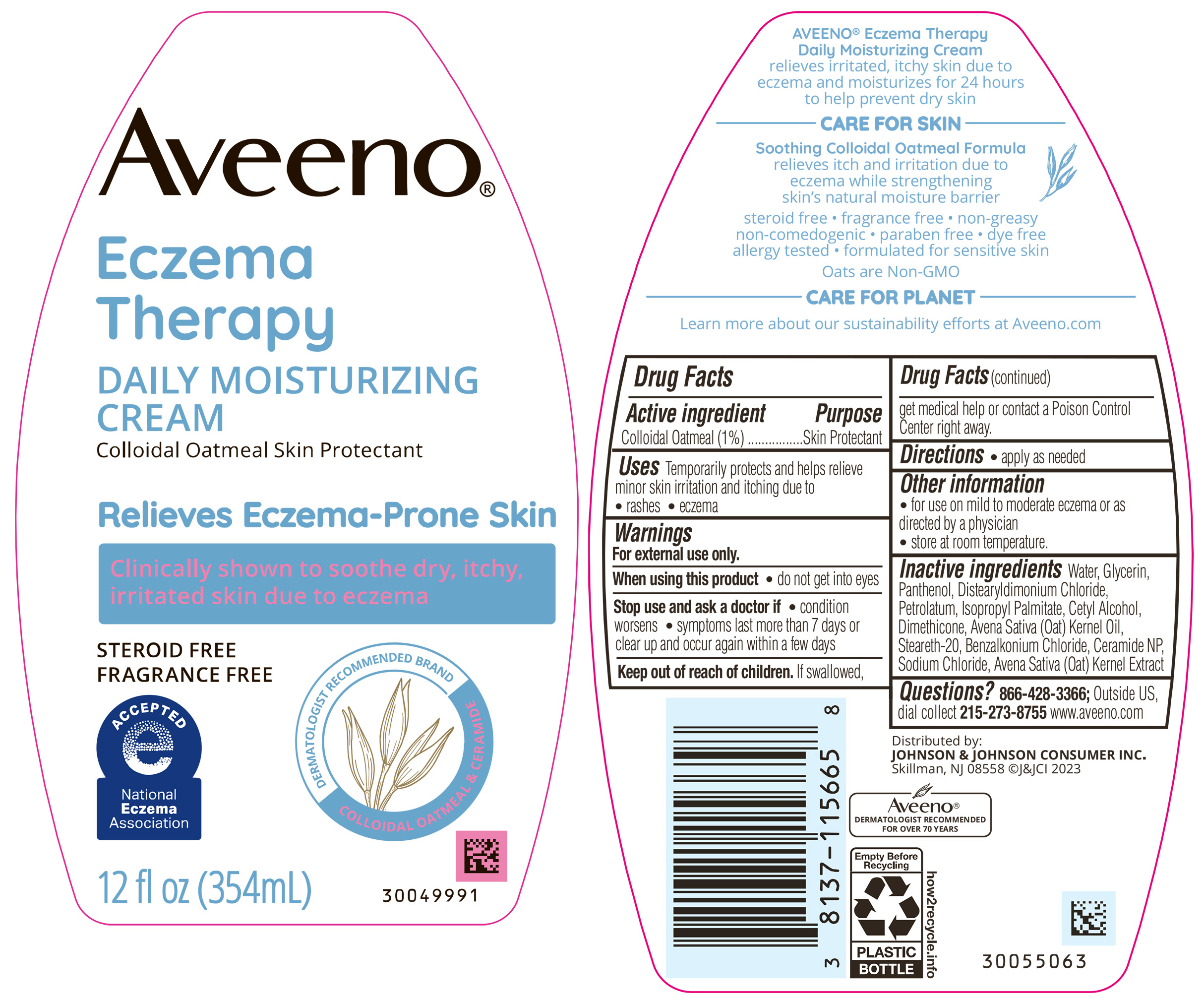
Uses
Temporarily protects and helps relieve minor skin irritation and itching due to
• rashes • eczema
Warnings
For external use only.
Other Information
- for use on mild to moderate eczema or as directed by a physician
- store at room temperature.
Inactive Ingredients
Water, Glycerin, Panthenol, Distearyldimonium Chloride, Petrolatum, Isopropyl Palmitate, Cetyl Alcohol, Dimethicone, Avena Sativa (Oat) Kernel Oil, Steareth-20, Benzalkonium Chloride, Ceramide NP, Sodium Chloride, Avena Sativa (Oat) Kernel Extract
PRINCIPAL DISPLAY PANEL - 354 mL Bottle
Aveeno ®
Eczema
Therapy
DAILY MOISTURIZING
CREAM
Colloidal Oatmeal Skin Protectant
Relieves Eczema-Prone Skin
Clinically shown to soothe dry, itchy,
irritated skin due to eczema
STEROID FREE
FRAGRANCE FREE
ACCEPTED
e
National
Eczema
Association
DERMATOLOGIST RECOMMENDED BRAND
COLLOIDAL OATMEAL & CERAMIDE
12 fl oz (354mL)