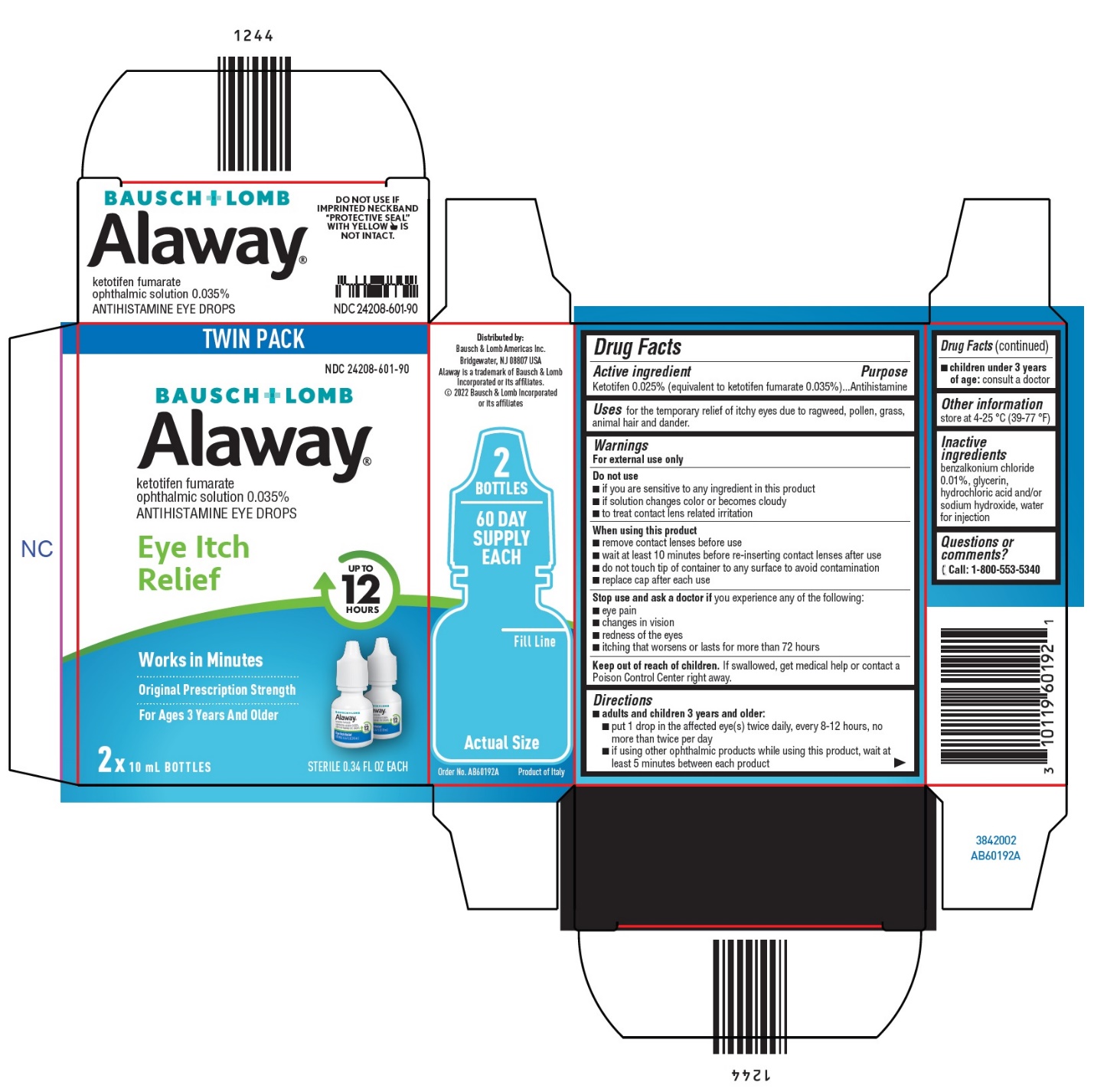
Warnings
For external use only
Do not use
- •
- if you are sensitive to any ingredient in this product
- •
- if solution changes color or becomes cloudy
- •
- to treat contact lens related irritation
When using this product
- •
- remove contact lenses before use
- •
- wait at least 10 minutes before re-inserting contact lenses after use
- •
- do not touch tip of container to any surface to avoid contamination
- •
- replace cap after each use
Directions
- •
-
adults and children 3 years and older:
- •
- put 1 drop in the affected eye(s) twice daily, every 8-12 hours, no more than twice per day
- •
- if using other ophthalmic products while using this product, wait at least 5 minutes between each product
- •
-
children under 3 years of age: consult a doctor
Inactive ingredients
benzalkonium chloride 0.01%, glycerin, hydrochloric acid and/or sodium hydroxide, water for injection
Package/Label Principal Display Panel
TWIN PACK
NDC 24208-601-90
BAUSCH + LOMB
Alaway®
ketotifen fumarate
ophthalmic solution 0.035%
ANTIHISTAMINE EYE DROPS
Eye Itch
Relief
UP TO
12
HOURS
Works in Minutes
Original Prescription Strength
For Ages 3 Years And Older
2x 10 mL BOTTLES
STERILE 0.34 FL OZ EACH
3842002
AB60192A