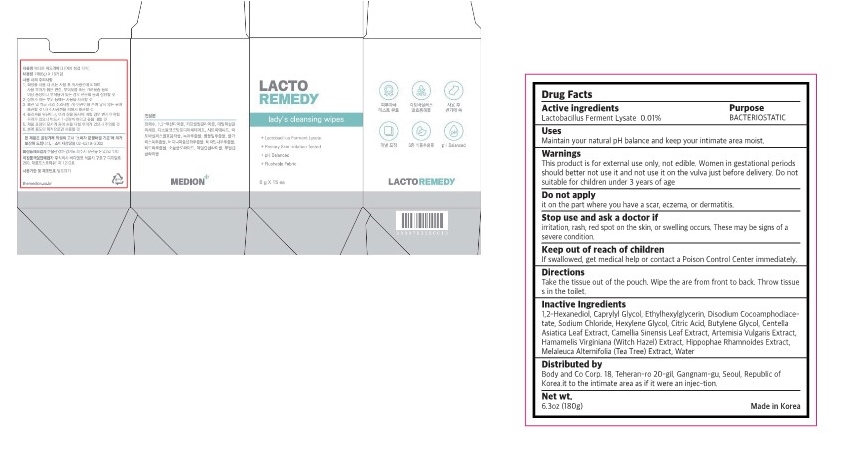
warnings
Do not suitable for children under 3 years of age.
Do not apply it on the part where you have a scar, eczema, or dermatitis.
warnings
Women in gestational periods should better not use it and not use it on the vulva just before delivery.
warnings
Stop use and ask a doctor if
irritation, rash, red spot on the skin, or swelling occurs. These may be signs of a severe condition.
warnings
Keep out of reach of children
If swallowed, get medical help or contact a Poison Control Center immediately
Directions
Take the tissue out of the pouch. Wipe the are from front to back. Throw tissue s in the toilet.
Inactive ingredient
1,2-Hexanediol, Caprylyl Glycol, Ethylhexylglycerin, Disodium Cocoamphodiace- tate, Sodium Chloride, Hexylene Glycol, Citric Acid, Butylene Glycol, Centella Asiatica Leaf Extract, Camellia Sinensis Leaf Extract, Artemisia Vulgaris Extract, Hamamelis Virginiana (Witch Hazel) Extract, Hippophae Rhamnoides Extract, Melaleuca Alternifolia (Tea Tree) Extract, Water