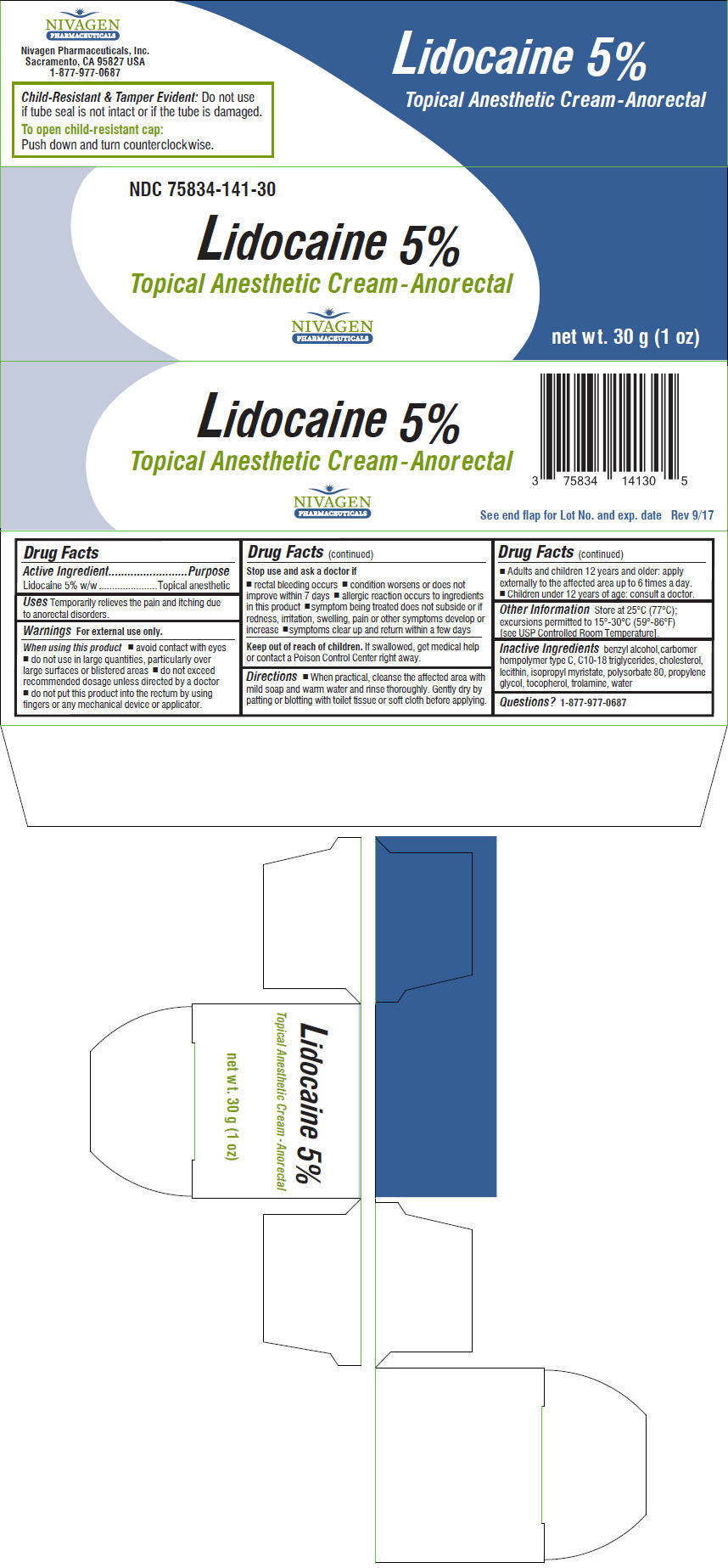
Warnings
For external use only.
When using this product
- avoid contact with eyes
- do not use in large quantities, particularly over large surfaces or blistered areas
- do not exceed recommended dosage unless directed by a doctor
- do not put this product into the rectum by using fingers or any mechanical device or applicator.
Stop use and ask a doctor if
- rectal bleeding occurs
- condition worsens or does not improve within 7 days
- allergic reaction occurs to ingredients in this product
- symptom being treated does not subside or if redness, irritation, swelling, pain or other symptoms develop or increase
- symptoms clear up and return within a few days
Directions
- When practical, cleanse the affected area with mild soap and warm water and rinse thoroughly. Gently dry by patting or blotting with toilet tissue or soft cloth before applying.
- Adults and children 12 years and older: apply externally to the affected area up to 6 times a day.
- Children under 12 years of age: consult a doctor.
Other Information
Store at 25°C (77°C); excursions permitted to 15°-30°C (59°-86°F) [see USP Controlled Room Temperature].