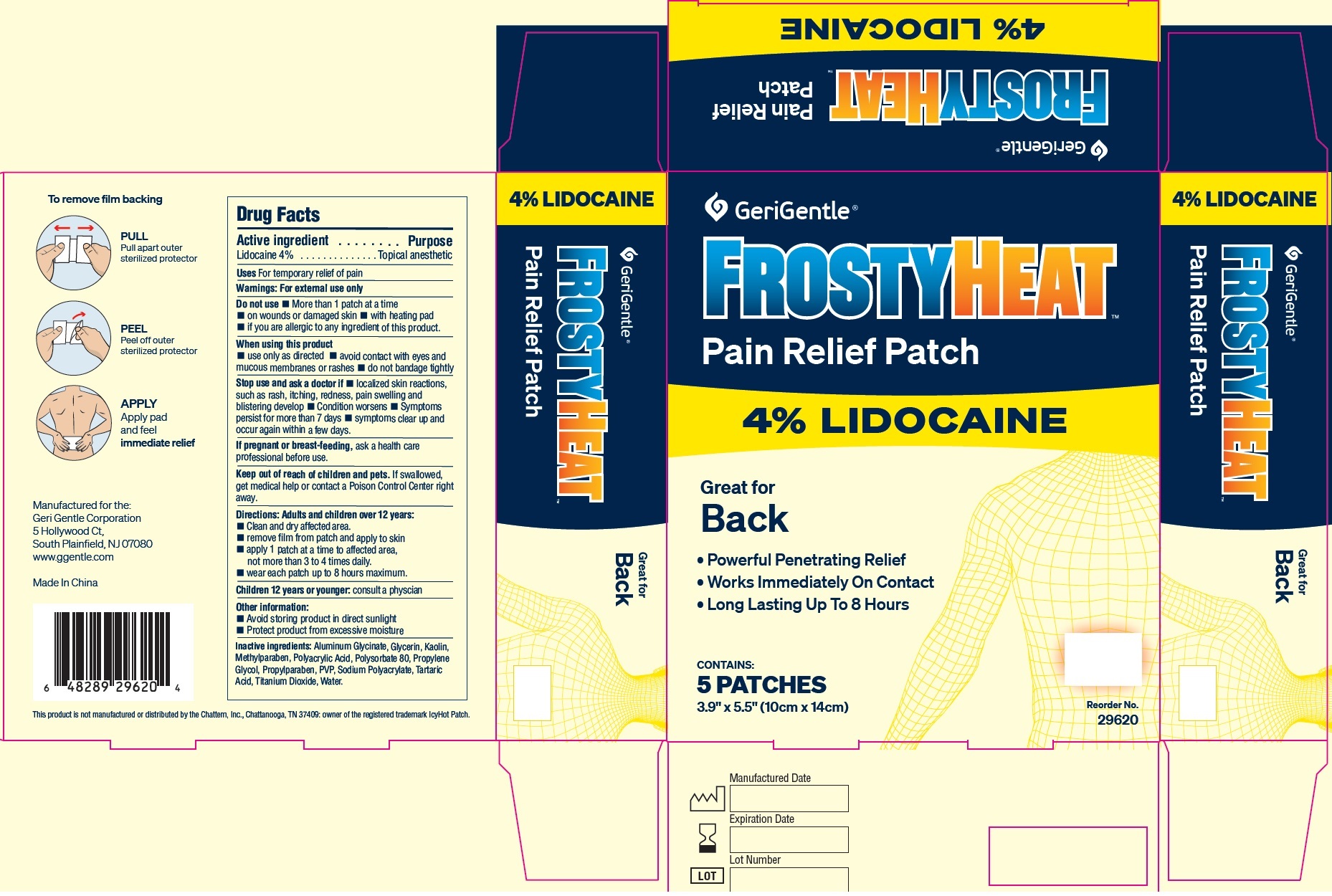
Active ingredient
Lidocaine 4%
Purpose
Topical anesthetic
Uses
For temporary relief of pain
Warnings:
For external use only
Do not use
- More than 1 patch at a time
- on wounds or damaged skin
- with heating pad
- if you are allergic to any ingredient of this product.
When using this product
- use only as directed
- avoid contact with eyes and mucous membranes or rashes
- do not bandage tightly
Stop use and ask a doctor if
- localized skin reactions, such as rash, itching, redness, pain swelling and blistering develop
- Condition worsens
- Symptoms persist for more than 7 days
- symptoms clear up and occur again within a few days.
If pregnant or breast-feeding,
ask a health care professional before use.
Keep out of reach of children and pets.
If swallowed, get medical help or contact a Poison Control Center right away.
Directions:
Adults and children over 12 years:
- Clean and dry affected area.
- remove film from patch and apply to skin
- apply 1 patch at a time to affected area, not more than 3 to 4 times daily.
- wear each patch up to 8 hours maximum.
- consult a physcian
Children 12 years or younger:
Other information:
- Avoid storing product in direct sunlight
- Protect product from excessive moisture
Inactive ingredients:
Aluminum Glycinate, Glycerin, Kaolin, Methylparaben, Polyacrylic Acid, Polysorbate 80, Propylene Glycol, Propylparaben, PVP, Sodium Polyacrylate, Tartaric Acid, Titanium Dioxide, Water.