DDM Ear Drops for Swimmers
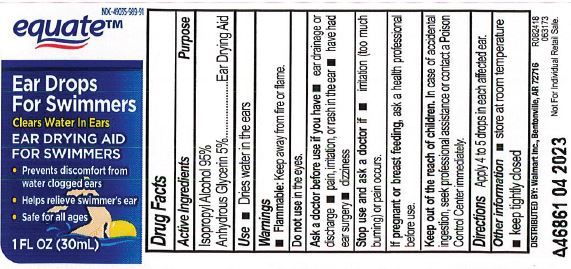
Drug Facts
Active Ingredient
Isopropyl Alcohol 95% in anhydrous glycerin
Use
Dries water in the ears.
Warnings
Flammable: Keep away from fire and flame.
Ask a doctor before use if you have
Ear drainage or discharge.
Pain, irritation, or rash in the ear.
Have had ear surgery.
Dizziness.
Stop use and ask doctor if
Irritation (too much burning) or pain occurs.
If pregnant or breast feeding,
ask a health professional before use.
Keep out of reach of children.
- In case of accidental ingestion, seek professional assistant or contact a Poison Control Center immediately.
Directions
Apply 4 to 5 drops in each affected ear.
Other information
Store at room temperature.
Inactive Ingredient
Glycerin
DDM

HEB

CVS


Leader

QC

PV

RA

Top Care

Equate