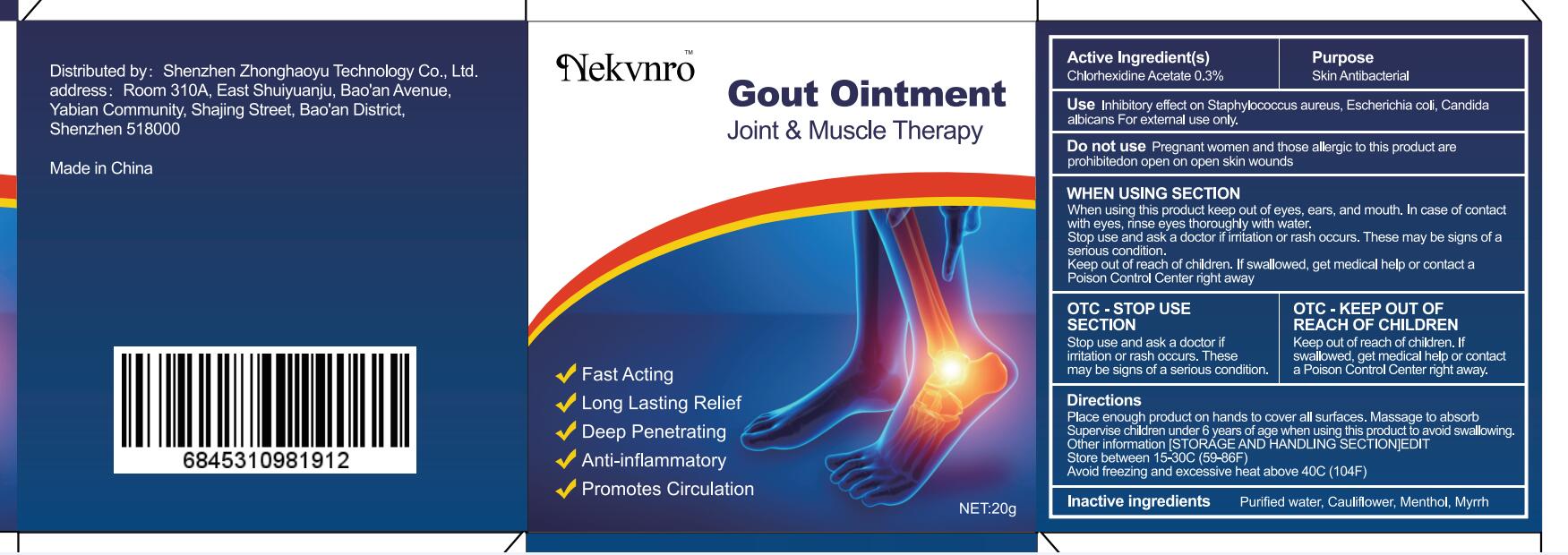
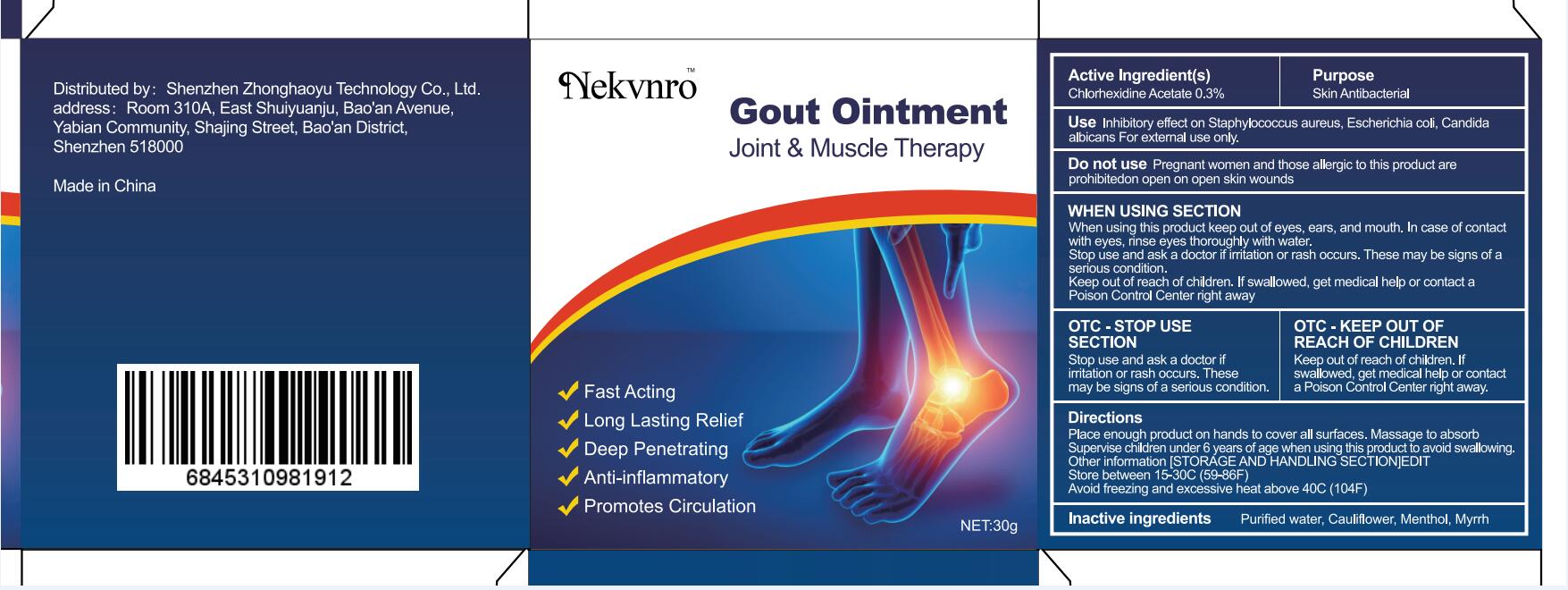
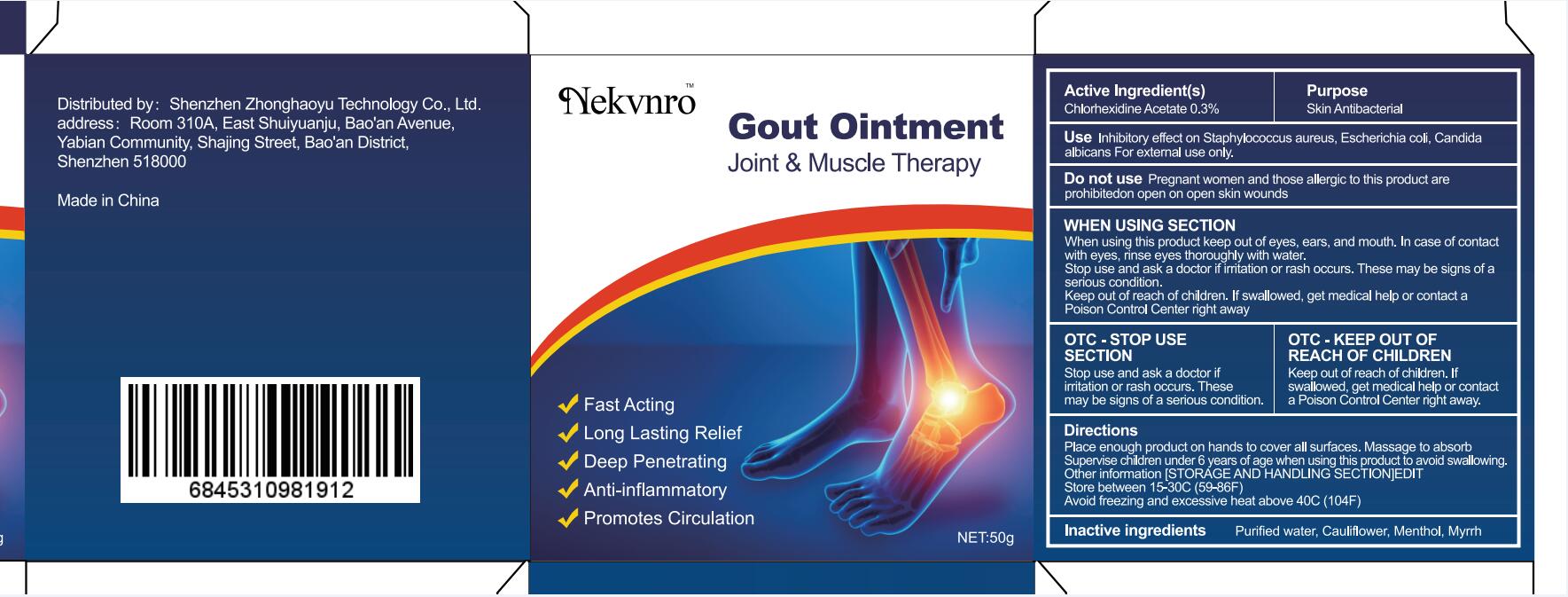
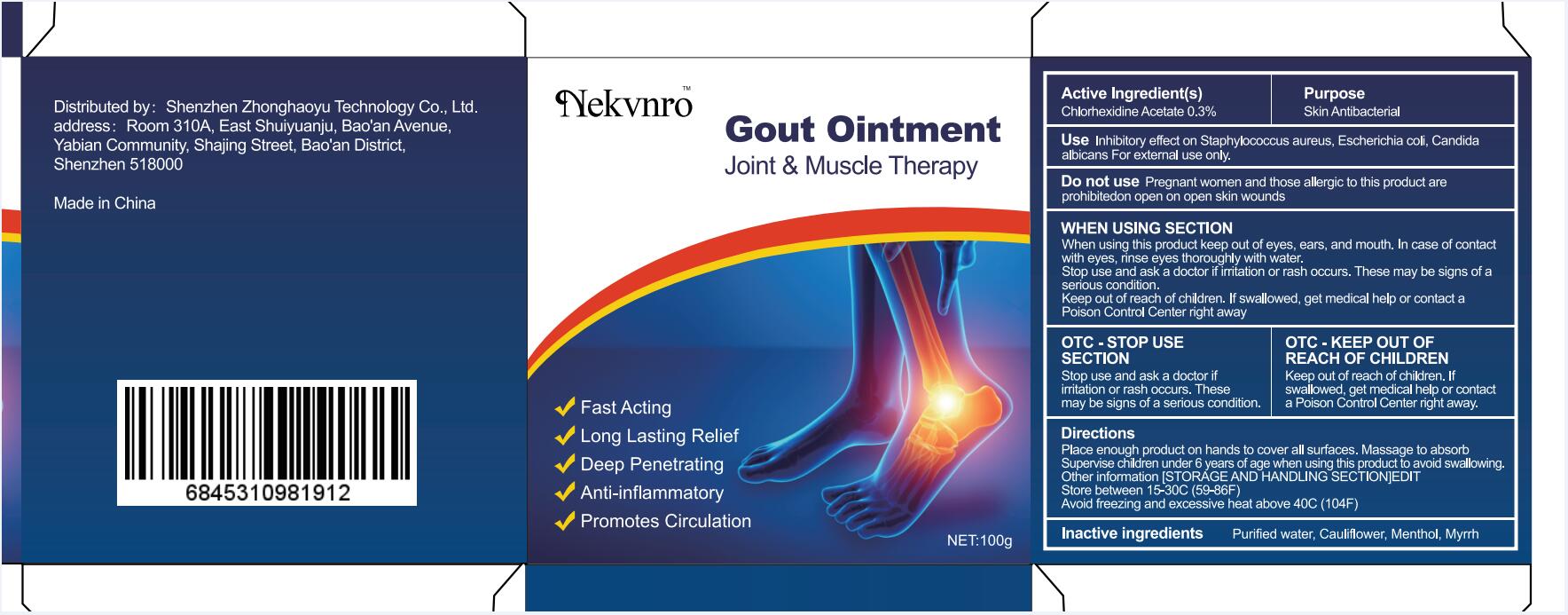
Use
Inhibitory effect on Staphylococcus aureus, Escherichia coli, Candida albicans For external use only.
Do not use
Pregnant women and those allergic to this product are prohibitedon open on open skin wounds
WHEN USING SECTION
When using this product keep out of eyes, ears, and mouth. In case of contact with eyes, rinse eyes thoroughly with water.
Stop use and ask a doctor if irritation or rash occurs. These may be signs of a serious condition.
Keep out of reach of children. If swallowed, get medical help or contact a Poison Control Center right away
OTC - STOP USE SECTION
Stop use and ask a doctor if iritation or rash occurs.These may be signs of a serious condition.
OTC - KEEP OUT OF REACH OF CHILDREN
Keep out of reach of children. If swallowed, get medical help or contac a Poison Control Center right away.
Directions
Place enough product on hands to cover all surfaces. Massage to absorb Supervise children under 6 years of age when using this product to avoid swallowing.
Other information
[STORÁGE AND HANDLING SECTION]EDIT
Store between 15-30C (59-86F)
Avoid freezing and excessive heat above 40C (104F)