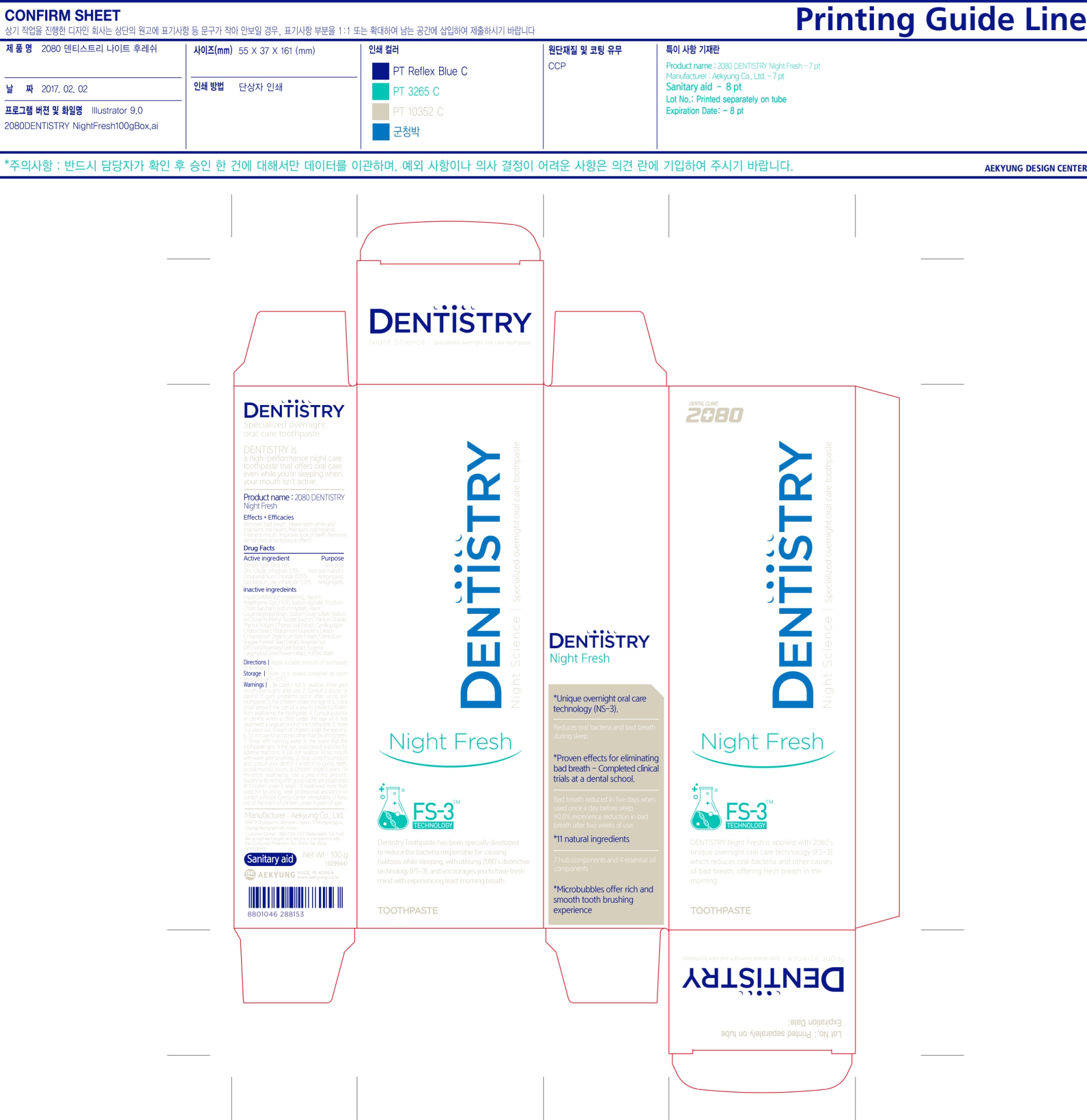
Be careful not to swallow. Rinse your mouth thoroughly after use. Consult a doctor or dentist if gum problems occur after using this toothpaste.
Stop using this product and consult your dentist if irritation to gums, teeth, or oral mucosa occurs
Do not swallow. Rinse mouth with water after brushing.
Children under 6 years:
- To minimize swallowing, use a pea-sized amount. Supervise brushing until good habits are established.
- If swallowed more than used for brushing, seek professional assistance or contact a Poison Control Center immediately.
Do not use for purpose other than brushing teeth. Rinse with running water in the event that the toothpaste gets in the eye and consult a doctor for adverse reactions
Customer Center: 080-024-1357 (Nationwide Toll-Free)
We accept exchanges and returns in compliance with the Consumer Protection Act of the Fair Trade Commission
www.aekyung.co.kr
Manufacturer: Aekyung Co., Ltd
1547-9 Chungui-ro, Jeongsan-myeon, Cheongyanggun, Chungcheongnam-do, Korea
Inactive Ingredients: Liquid Sorbitol (Non-crystalizing), Glycerin, Polyethylene Glycol 1500, Sodium Alginate, Trisodium Citrate, Saccharin Sodium Hydrate, Cocamidopropyl Betain, Sodium Lauryl Sulfate, Sodium N-Cocoyl-N-Methyl Taurate Solution, Titanium Dioxide, Thymus Vulgaris (Thyme) Leaf Extract, Cymbopogon Citratus Extract, Pelargonium Graveolens Extract, Cinnamonium Zeylanicum Bark Extract, Foenlculum Vulgare (Fennel) Seed Extract, Rosemarinus Officinalis (Rosemary) Leaf Extract, Eugenia Caryphyllus (Clove) Flower Extract, Purified Water