CYSTEX PLUS- methenamine, sodium salicylate tablet
DSE Healthcare Solutions, LLC
Disclaimer: This drug has not been found by FDA to be safe and effective, and this labeling has not been approved by FDA. For further information about unapproved drugs, click here.
----------
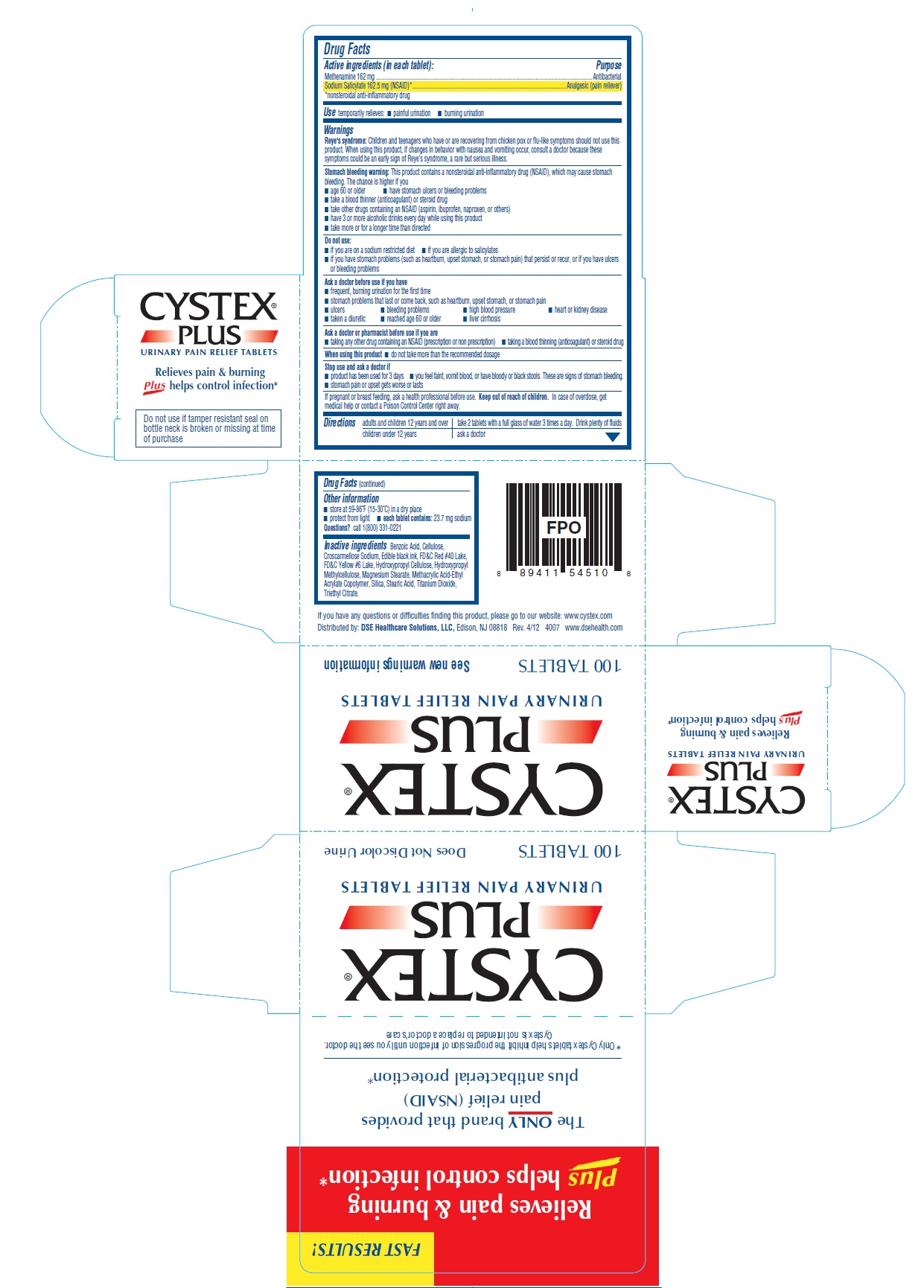
Drug Facts
Active ingredients (in each tablet):
Methenamine 162 mg
Sodium Salicylate 162.5 mg (NSAID)*
*nonsteroidal anti-inflammatory drug
Warnings
Reye's syndrome: Children and teenagers who have or are recovering from chicken pox or flu-like symptoms should not use this product. When using this product, if changes in behavior with nausea and vomiting occur, consult a doctor because these symptoms could be an early sign of Reye's syndrome, a rare but serious illness.
Stomach bleeding warning: This product contains a nonsteroidal anti-inflammatory drug (NSAID), which may cause stomach bleeding. The chance is higher if you
-
age 60 or older
-
have stomach ulcers or bleeding problems
-
take a blood thinner (anticoagulant) or steroid drug
-
take other drugs containing an NSAID (aspirin, ibuprofen, naproxen, or others)
-
have 3 or more alcoholic drinks every day while using this product
-
take more or for a longer time than directed
Do not use:
-
if you are on a sodium restricted diet
-
if you are allergic to salicylates
-
if you have stomach problems (such as heartburn, upset stomach, or stomach pain) that persist or recur, or if you have ulcers or bleeding problems
Ask a doctor before use if you have
-
frequent, burning urination for the first time
-
stomach problems that last or come back, such as heartburn, upset stomach, or stomach pain
-
ulcers
-
taken a diuretic
-
bleeding problems
-
reached age 60 or older
-
high blood pressure
-
liver cirrhosis
-
heart or kidney disease
Ask a doctor or pharmacist before use if you are
-
taking any other drug containing an NSAID (prescription or non prescription)
-
taking a blood thinning (anticoagulant) or steroid drug
Stop use and ask a doctor if
-
product has been used for 3 days
-
you feel faint, vomit blood, or have bloody or black stools. These are signs of stomach bleeding.
-
stomach pain or upset gets worse or lasts
Keep out of reach of children.
In case of overdose, get medical help or contact a Poison Control Center right away.
Directions
|
adults and children 12 years and over |
take 2 tablets with a full glass of water 3 times a day. Drink plenty of fluids |
|
children under 12 years |
ask a doctor |
Other information
-
store at 59-86°F (15-30°C) in a dry place
-
protect from light
- each tablet contains: 23.7 mg sodium
Inactive ingredients
Benzoic Acid, Cellulose,Croscarmellose Sodium, Edible black ink, FD&C Red #40 Lake,FD&C Yellow #6 Lake, Hydroxypropyl Cellulose, Hydroxypropyl Methylcellulose, Magnesium Stearate, Methacrylic Acid-Ethyl Acrylate Copolymer, Silica, Stearic Acid, Titanium Dioxide,Triethyl Citrate.
CYSTEX®
PLUS
URINARY PAIN RELIEF TABLETS
FAST RESULTS!
100 Tablets See New Warnings Information
Does Not Discolor Urine
Relieves pain & burning
Plus helps control infection*
The ONLY brand that provides
pain relief (NSAID)
plus antibacterial protection*
*Only Cystex tablets help inhibit the progression of infection until you see the doctor.
Cystex is not intended to replace a doctor’s care
Do not use if tamper resistant seal on
bottle neck is broken or missing at time
of purchase
If you have any questions or difficulties finding this product, please go to our website: www.cystex.com
Distributed by: DSE Healthcare Solutions, LLC, Edison, NJ 08818 Rev. 4/12 4007 www.dsehealth.com
| CYSTEX
PLUS
methenamine, sodium salicylate tablet |
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
| Labeler - DSE Healthcare Solutions, LLC (603016069) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| ULTRAtab Laboratories, Inc. | 151051757 | MANUFACTURE(42364-4501) | |