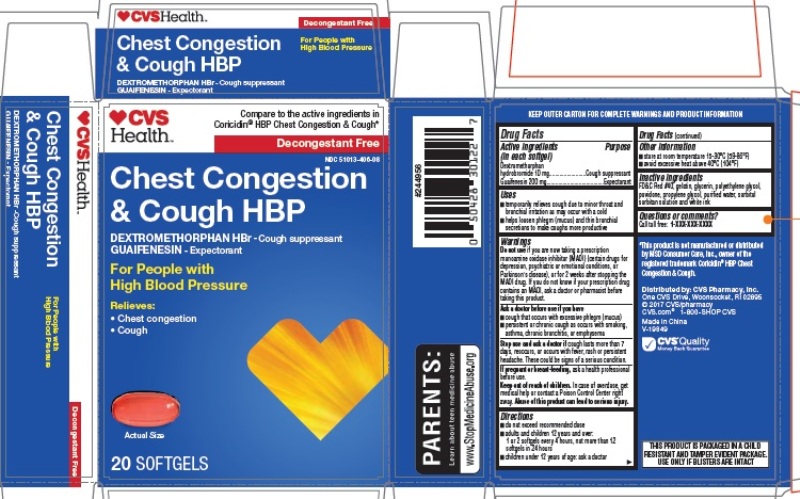
CHEST CONGESTION AND COUGH HBP- dextromethorphan hydrobromide, guaifenesin capsule, liquid filled
PuraCap Pharmaceutical LLC
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
Active ingredient (in each softgel)
Dextromethorphan hydrobromide 10 mg
Guaifenesin 200 mg
Purpose
Cough suppressant
Expectorant
Uses
- temporarily relieves cough due to minor throat and bronchial irritation as may occur with a cold
- helps loosen phlegm (mucus) and thin bronchial secretions to make coughs more productive
Warnings
Do not use if you are now taking a prescription monoamine oxidase inhibitor (MAOI) (certain drugs for depression, psychiatric or emotional conditions, or Parkinson's disease), or for 2 weeks after stopping the MAOI drug. If you do not know if your prescription drug contains an MAOI, ask a doctor or pharmacist before taking this product.
Ask a doctor before use if you have
- cough that occurs with excessive phlegm (mucus)
- persistent or chronic cough as occurs with smoking, asthma, chronic bronchitis, or emphysema
Stop use and ask a doctor if cough lasts more than 7 days, reoccurs, or occurs with fever, rash or persistent headache. These could be signs of a serious condition.
If pregnant or breast-feeding, ask a health professional before use.
Keep out of reach of children. In case of overdose, get medical help or contact a Poison Control Center right away. Abuse of this product can lead to serious injury.
Directions
- do not exceed recommended dose
- adults and children 12 years and over:
1 or 2 softgels every 4 hours, not more than 12 softgels in 24 hours
- children under 12 years of age: ask a doctor
Other information
- store at room temperature 15-30°C (59-86°F)
- avoid excessive heat above 40°C (104°F)
Inactive ingredients
FD&C Red #40, gelatin, glycerin, polyethylene glycol, povidone, propylene glycol, purified water, sorbitol sorbitan solution and white ink
Questions or comments
Call toll free: 1-855-215-8180
Principal Display Panel
CVS Chest Congestion & Cough HBP 20 SOFTGELS
NDC: 51013-406-08
*Compare to the active ingredients in Coricidin® HBP Chest Congestion & Cough