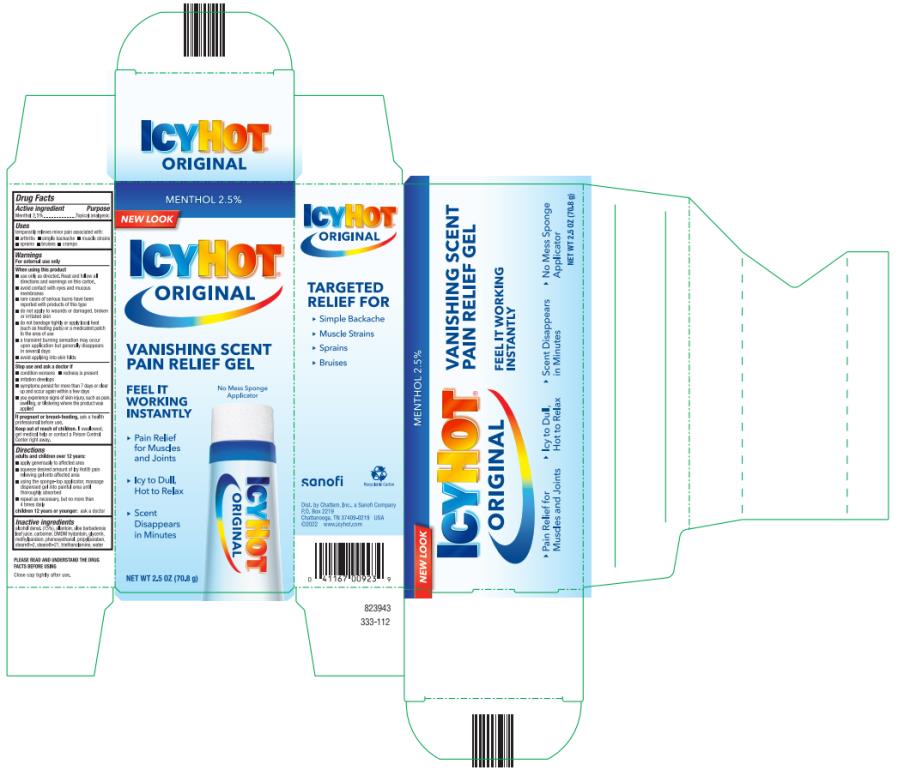
Uses
temporarily relieves minor pain associated with:
- arthritis
- simple backache
- muscle strains
- sprains
- bruises
- cramps
Warnings
For external use only
When using this product
■ use only as directed. Read and follow all directions and warnings on this carton.
■ avoid contact with eyes and mucous membranes
■ rare cases of serious burns have been reported with products of this type
■ do not apply to wounds or damaged, broken or irritated skin
■ do not bandage tightly or apply local heat (such as heating pads) or a medicated patch to the area of use
■ a transient burning sensation may occur upon application but generally disappears in several days
■ avoid applying into skin folds
Directions
adults and children over 12 years:
- apply generously to affected area
- squeeze desired amount of Icy Hot® pain relieving gel onto affected area
- using the sponge-top applicator, massage dispensed gel into painful area until thoroughly absorbed
- repeat as necessary, but not more than 4 times daily
children 12 years or younger: ask a doctor