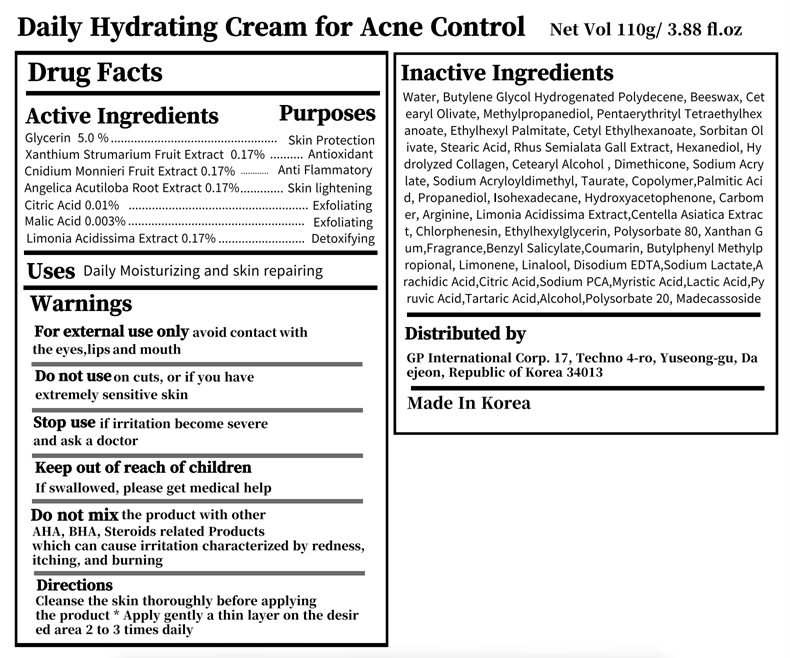
Warnings
Do not use on cuts, or if you have extremely sensitive skin
Do not mix the product with other AHA, BHA, Steroids related Products
which can cause irritation characterized by redness,
itching, and burning
Directions
Directions Cleanse the skin thoroughly before applying
the product * Apply gently a thin layer on the desired area 2 to 3 times daily
active ingredient
Glycerin 5.0 % Skin Protection
Xanthium Strumarium Fruit Extract 0.17% Antioxidant
Cnidium Monnieri Fruit Extract 0.17% Anti Flammatory
Angelica Acutiloba Root Extract 0.17% Skin lightening
Citric Acid 0.01% Exfoliating
Malic Acid 0.003% Exfoliating
Inactive Ingredients
Water, Butylene Glycol Hydrogenated Polydecene, Beeswax, Cetearyl Olivate, Methylpropanediol, Pentaerythrityl Tetraethylhexanoate, Ethylhexyl Palmitate, Cetyl Ethylhexanoate, Sorbitan Olivate, Stearic Acid, Rhus Semialata Gall Extract, Hexanediol, Hydrolyzed Collagen, Cetearyl Alcohol , Dimethicone, Sodium Acrylate, Sodium Acryloyldimethyl, Taurate, Copolymer,Palmitic Acid, Propanediol, Isohexadecane, Hydroxyacetophenone, Carbomer, Arginine, Limonia Acidissima Extract,Centella Asiatica Extract, Chlorphenesin, Ethylhexylglycerin, Polysorbate 80, Xanthan Gum,Fragrance,Benzyl Salicylate,Coumarin, Butylphenyl Methylpropional, Limonene, Linalool, Disodium EDTA,Sodium Lactate,Arachidic Acid,Citric Acid,Sodium PCA,Myristic Acid,Lactic Acid,Pyruvic Acid,Tartaric Acid,Alcohol,Polysorbate 20, Madecassoside