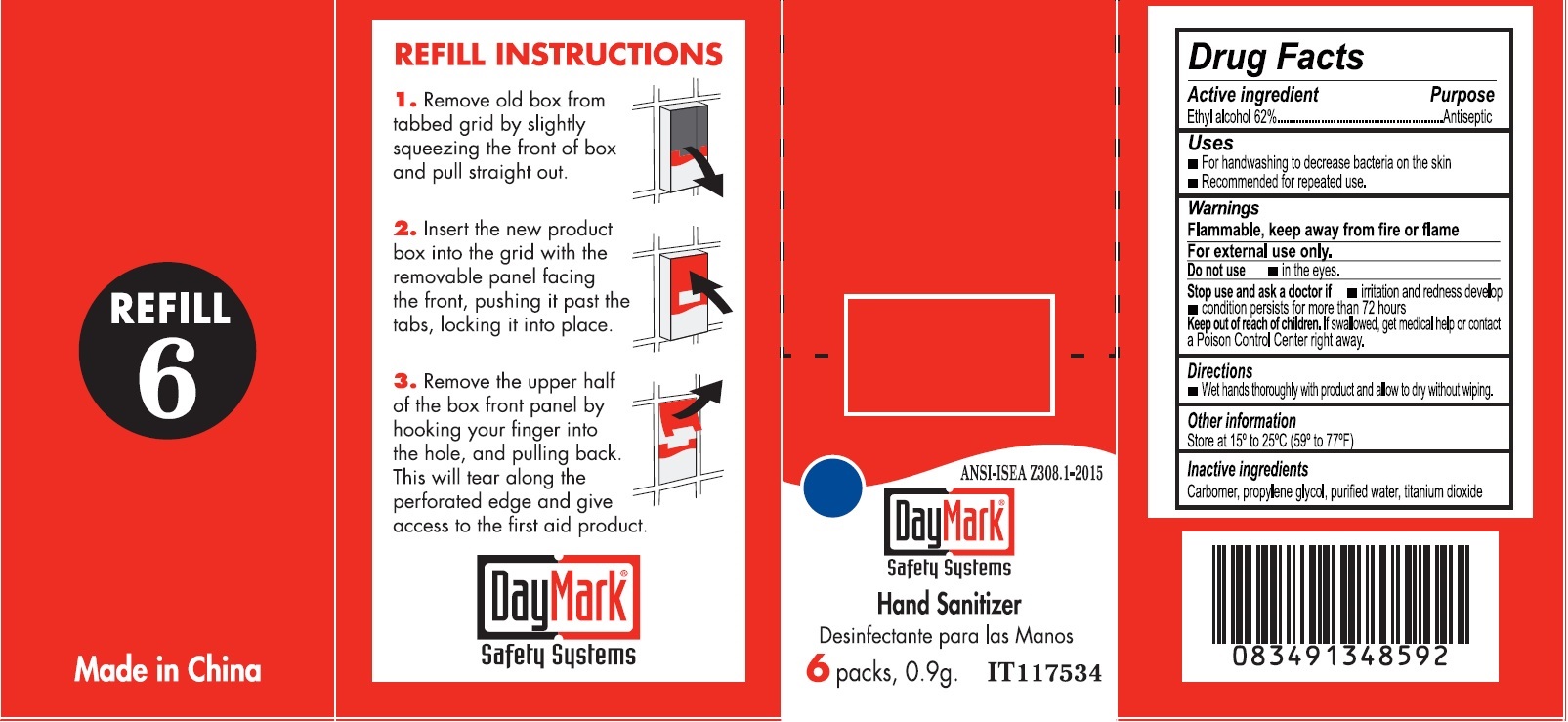
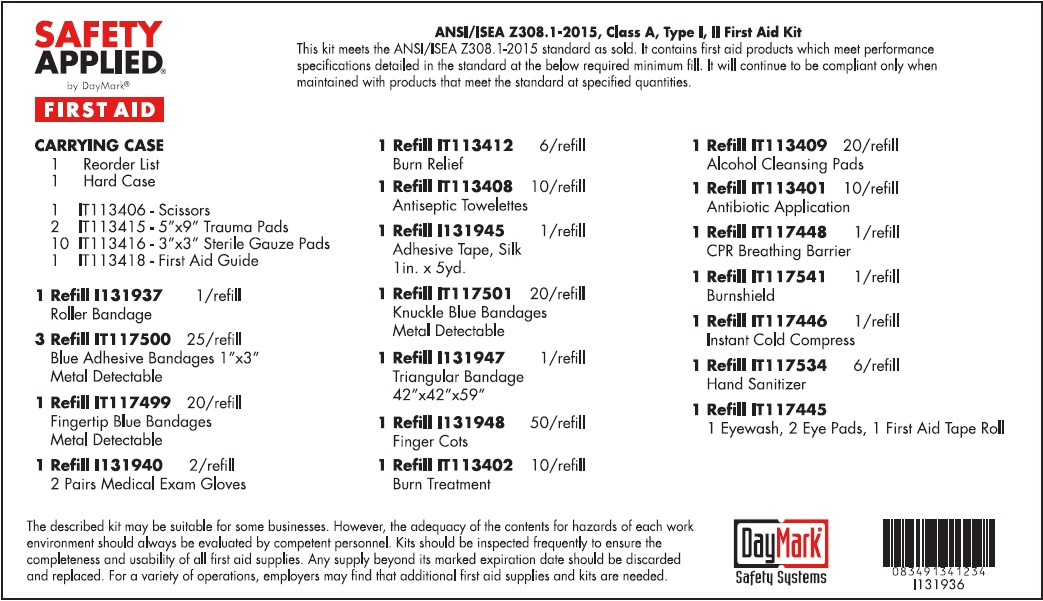
Warnings
For external use only.
When using this product
• to avoid contamination, do not touch tip of container to any surface • do not reuse • once opened, discard • obtain immediate medical treatment for all open wounds in or near the eyes
Directions
• Flush the affected eye as needed, controlling the rate of flow of solution by pressure on the bottle
Other information
• not for use as contact lens solution
• use before expiration date marked on the bottle
• store at room temperature, 5° to 35°C (41° to 95°F)
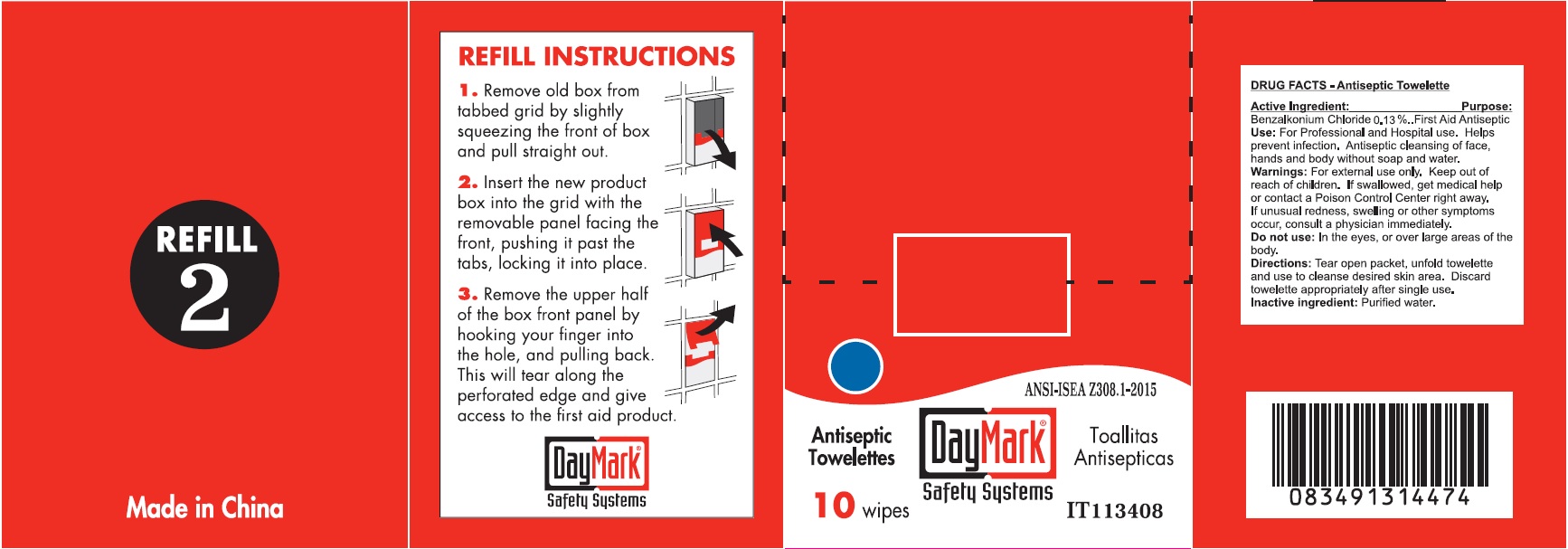
Use:
For Professional and Hospital use. Helps prevent infection. Antiseptic cleansing of face, hands and body without soap and water.
Warnings:
For external use only.
Directions:
Tear open packet, unfold towelette and use to cleanse desired skin area. Discard towelette appropriately after single use.
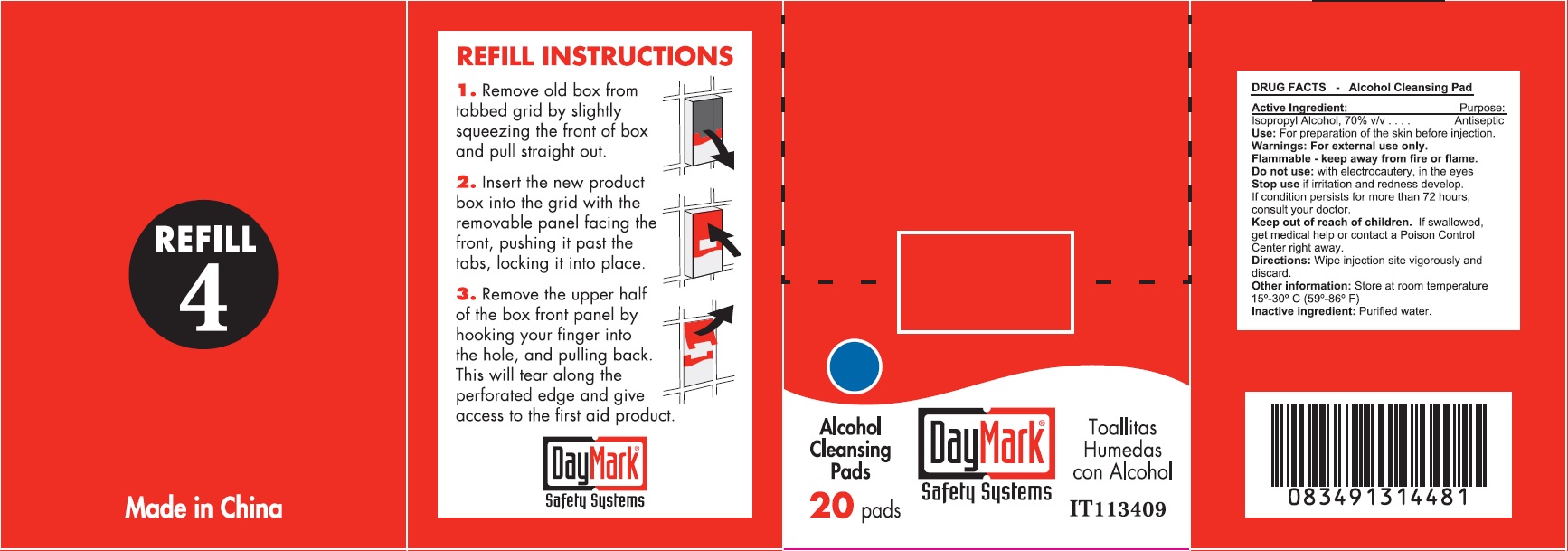
Warnings:
For external use only.Flammable - keep away from fire or flame.
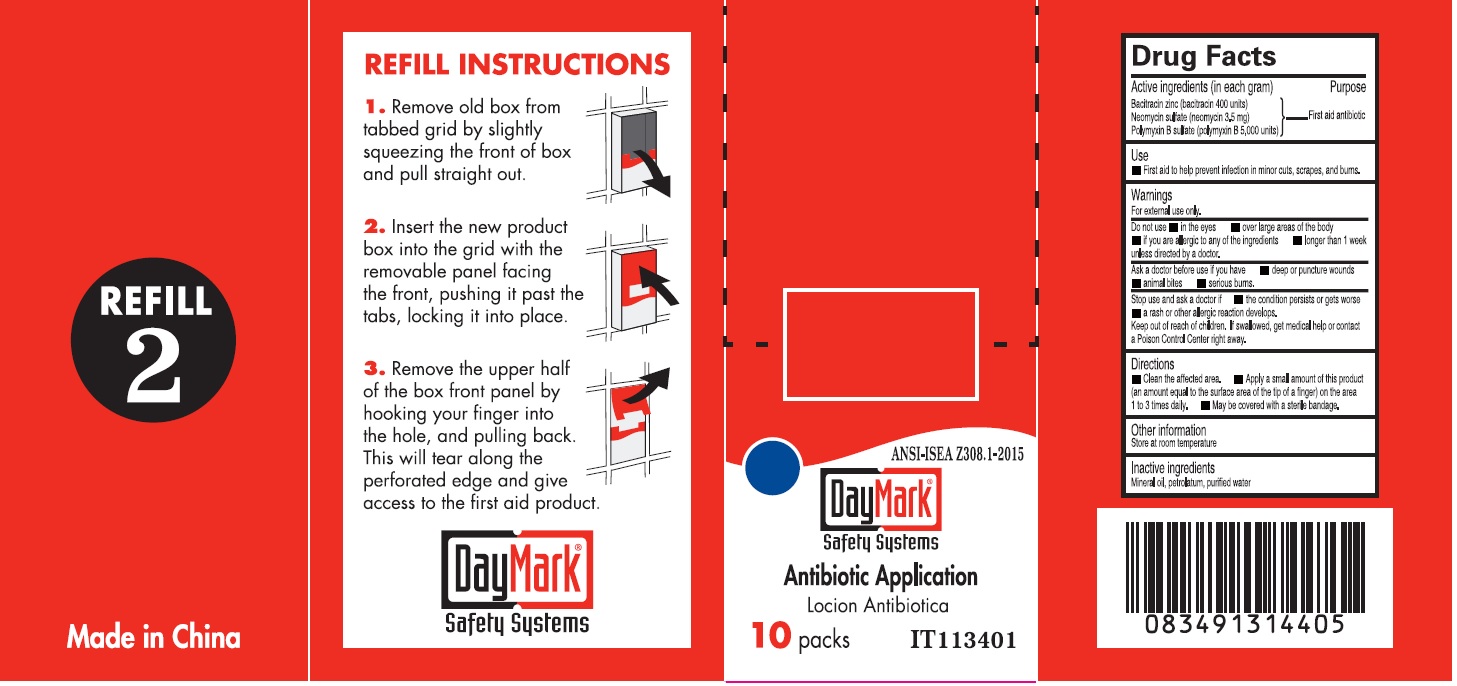
Active ingredients (in each gram)
Bacitracin zinc (bacitracin 400 units) Neomycin sulfate (neomycin 3.5mg) Polymyxin B sulfate (polymyxin B 5,000 units)
Warnings
For external use only
Do not use
• in the eyes • over large areas of the body • if you are allergic to any of the ingredient • longer than 1 week unless directed by a doctor.
Directions
• Clean the affected area. • Apply a small amount of this product (an amount equal to the surface area of the tip of a finger) on the area 1 to 3 times daily. • May be covered with a sterile bandage.
Uses
- First aid to help prevent infection in minor cuts, scrapes, and burns.
- For the temporary relief of pain and itching associated with minor burns , minor cuts, and scrapes
Warnings
For external use only.
Do not use
• in the eyes • over large areas of the body • in large quantities • over raw surfaces or blistered areas • longer than 1 week unless directed by a doctor
Directions
- Clean the affected area
- Adults and children 2 years of age and older: Apply a small amount of this product to affected area not more than 3 times daily
- Children under 2 years of age: consult a doctor
- May be covered with a sterile bandage